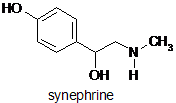
Risks from Bitter Orange Products (Citrus aurantium).
Copyright © Tony Burfield July 2005.
Pre-amble.
Several aromatherapists have asked about information concerning any risks from synephrine from certain commodities containing extracts of sweet orange Citrus sinensis L. or bitter orange Citrus aurantium L., and from bitter orange extract concentrate, which is being marketed to aromatherapists and natural perfumers.
Synephrine from the green unripe peels of Citrus aurantium L. (bitter orange) together with other naturally occurring adrenergic amines such as N-methyltyramine, are known to present ephedrine-like effects. However it has been suggested that whilst epehedrine is a non-selective sympathomimetic (stimulating the sympathetic nervous system), synephrine is a semi-selective sympathomimetic, targeting other tissues (e.g. fatty tissues) as well as, or instead of, the heart. It is especially present in bitter orange extract used in flavourings and herbal extracts. Occurrence of synephrine in other varieties of orange peel such as the Seville orange is also known, and it has been stated that synephrine occurs to a greater or lesser extent in most citrus products.
Bitter orange peel concrete is commercially available, and is seen as a dark orange-brown very viscous liquid, with a striking tangy sweet marmalade character, with a slight honey back-note. Thirty-six hour dry-out is still distinctly orange like, but in a cooked/stewed orange way, having lost the fine top notes. There is also a hint of a burnt brown sugar note. The product finds potential application in eau fraiche perfume types, citrus blends and to give an interesting colouring to masculine fragrances. An absolute prepared from this material may be more useful (Burfield 2005). The synephrine content of this material is unknown.
It is also reported in the literature that ingestion of large amounts of orange peel (bitter or sweet) by children has caused intestinal colic, convulsions and even death (Jiangsu, through Leung & Foster 1996).
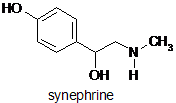
Ephedrine alkaloid containing supplements were banned by the FDA on April 12th 2004 on the basis that they present an unreasonable risk of illness or injury, having been linked to significant adverse health effects, including heart attack and stroke – see http://www.fda.gov/oc/initiatives/ephedra/february2004/. It is arguably the case that formulations for weight loss products incorporating bitter orange extracts increased in popularity after the ephedra ban, although Chinese medicinal products such as zhishi and zhiqiao have been widely available for a considerable time, and contain quantifiable levels of orange peel extracts.
Media attention
In 1999 Herbalgram featured a letter by Italian 3 doctors (see Firenzuoli et al. 1999) giving an example of a ‘further example of a herbal preparation dangerous to humans’. Their communiqué suggests that, based on their animal experiments, a Citrus aurantium extract contained in the Chinese preparations zhishi and zhiqiao was dangerous to human health. Dharmananda of the Institute for Traditional Medicine, Portland, Oregon argues (see: http://www.itmonline.org/arts/syneph.htm#figure%201) that the preparation used for the experiments by Firenzuoli et al. was special in many respects, unlike traditional herbal medicine preparations, by containing some 6% synephrine – a factor of some 25 times that expected in normal Chinese citrus herb products. Further, the toxic reactions seemed unusual to Dharmananda given the stated dose, and the author attempts to explain this in terms of other mitigating factors.
A further paper on possible risks from Citrus aurantium extracts in dietary supplements was written by Fugh-Berman and Myers and featured in the journal Experimental Biology and Medicine (Fugh-Bergman & Myers 2004) the review suggesting that consumers should avoid dietary supplements containing this ingredient. This article was the subject of an interview with Mark Bluemthal the ABC founder by Barret-Mann in the Health section of Washington Post, and the above story can be followed at http://www.herbalgram.org/default/asp?c=borange. The bottom line according to Blumenthal is that injected synephrine and bitter orange extract show a rise in blood pressure, but this does not occur with oral use. Since then a rash of articles on the subject (largely coming to different conclusions) have appeared on various internet websites.
It should also be noted that there is no fully comprehensive review on Citrus aurantium products. The best collection of material I have seen on the subject is in French on the Réseau Proteus internet website (see ‘Further Reading’ below), and I strongly recommend this feature to you all.
My view is that any effects of synephrine could be considered 'adverse' if the subject receiving the material (say in the form of a prescribed Chinese medicine for weight loss) is unaware that there is a possibility that the drug could cause uncomfortable physiological effects - which still appears to have been the case in a limited number of reported instances, although other factors may be operating here as mentioned by Dharmananda above. It would also appear to this author, that considering the already established history of aromatherapeutic use, and on the present evidence, the likelihood of adverse physiological symptoms from synephrine occurring from topical use of commercial bitter or sweet orange oils as applied in a vegetable oil vehicle at a concentration of 0.5 to 2.5% (as in a normal aromatherapy body massage) is minimal. Perhaps however, in view of this information, we should be open-minded and look a little closer at the use of unripe/partially ripe citrus fruit oils - I am especially thinking of commodities like the expressed oil of green and yellow mandarins from Citrus reticulata Blanco var. mandarin).
References.
Burfield, T. (2005) form the forthcoming 2nd edn of Natural Aromatic Materials – Odours and Origins (1st edn pub. AIA Tampa USA).
Firenzuoli F, Calapai G, and Gori L (1999) “Physicians discuss orange extract” (letter), Herbalgram 46, 76-77.
Fugh-Bergman A. & Myers A. (2004) “Citrus aurantium, an ingredient of dietary supplements marketed for weight loss: current status of clinical and basic research, experimental biology and medicine” Experimental Biology and Medicine 229(8), 698-704.
Jiangsu (1996): Jiangsu Institute of Modern Medicine/Provincial Institute of Botany through “Orange (Bitter & Sweet)” monograph in Leung A.Y. & Foster S. (eds) Encyclopedia of Common Natural Ingredients used in Food, Drugs and Cosmetics 2nd edn pub. John Wiley p 393-397.
Further Reading.
Colker, C.M., Kalman, D.S., Torina, G.C., Perlis, T. and Street, C. (1999) “Effects of Citrus aurantium Extract, Caffeine, and St John's Wort on Body Fat Loss, Lipid Levels, and Mood States in Overweight Healthy Adults.” Cur. Ther. Res. 60, 145-153
http://www.reseauproteus.net/fr/Solutions/PlantesSupplements/Fiche.aspx?doc=orange_amere_ps and associated references.