Cropwatch
Issue 3.
Copyright Tony Burfield May 2004
1. Blue
Cypress oil [ Callitris intratropica Benth. et Hook f.]
In keeping with our follow up investigations on
failed get-rich-quick plantation schemes in Australia, I am thankful to Jim
Gobert for alerting me to another story, which has, allegedly, lost investors
millions. Blue cypress essential oil is produced by a special process from the
heartwood, wood, and bark of the North Cypress Pine -
a member of the Southern Conifer group of the Cupressaceae family, which grows
to 45m. and has fragrant wood. It is one of a number of native cypress trees
which grow in the aboriginal lands of Australia (e.g. the northerly Bathurst and
Melville Islands), but its geographic isolation has prevented its commercial
exploitation for a considerable time. Aboriginal uses of the resin from
Callitris intratropica include employment as glue and for contraceptive uses (Bowman
& Harris 1995). It was widely promoted in the essential oils and
aromatherapy professions a decade past. It can be steam distilled from the
heartwood/wood/bark, but a solvent extracted “oil”
is also available, appearing as a deep blue-black, highly coloured
mobile liquid, where much was previously made of its azulene-like properties.
Properties
However
Cropwatch’s opinion is that it is hard to see why the oil should be
particularly attractive to perfumers. Burfield (2000) describes the oil as
follows: “In colder weather the oil may become semi-, or almost completely,
solid (presumably due to the guaiol content). The odour
is overwhelmingly woody, and slightly earthy, the top-note being multi-faceted,
with the following aspects being discernable: there is a medicinal almost ylang-like
note, a minor pine-like quality, a touch of spiciness and a pineapple-like
fruitiness. After a few minutes the odour profile becomes piney-resinous,
loosing some dryness and becoming sweeter. The dry-out
is woody-earthy and celery-like.” It isn’t regarded by the author as
particularly interesting perfumery material, but it may have a certain novelty
ingredient status.
The
composition of the oil is summarized again by Burfield (2000): “The oil
contains sesquiterpenes such as b-elemene
and d-selinene
and sesquiterpene alcohols such as guaiol (26%) and b-eudesmol
(6.3%). The blue colouring may be ascribed, at least in part, to the presence of
guaiazulene (1.6%) although other complex structures with a resemblance to the
azulene moiety are present.


In
a (somewhat strange) attempt to compare the oil with other commercial oils, some
attention has been drawn to an alleged similarity with another guaiol containing
oil: guaiacwood oil, although the sweetness of guaiacwood oil is not
particularly mirrored in this oil. However the oil has been ‘image marketed’
in the cosmetics trade on the fact that it is the only wood oil containing
guaiazulene which has alleged anti-bacterial properties (although a more
cost-effective and more ecologically sound plant source of guaiazulene might be
German chamomile oil). Bowles (2000)
previously set out the oil’s history & chemistry,
as well as outlining the uses of the oil, and its anti-inflammatory,
anti-irritant and anti-viral effects. The author’s experience of the oil has
been less upbeat, centering around poor keeping quality and unacceptable batch
to batch variability.
Legal
wrangles
The
Age, an
Australian newspaper, carried a story (“Bitter Blue”) on April 21, 2004,
described a legal wrangle over patents and allegations of deception. The battle
is described as being between Mike Collins who claims to have discovered the oil
first, and Bill McGilvray, well known essential oil producer, and former
president of the
Australian Tea-Tree Industry Association. The article describes a
decision taken by the
Delegate
of the Commissioner of Patents
in June 2002,
ruling that
McGilvray should loose the rights to log
the trees on Aboriginal and on Crown land for allegedly breaching contracts and
failing to pay royalties according to the Government and spokesmen of the Tiwi
aboriginal people.
It is further reported in the article that seven South African investors lost
$100,000 in the wrangle. You can read the full story at http://www.theage.com.au/articles/2004/04/20/1082395850945.html
References:
Bowles
J. (2000) Simply Essential. Aug 2000.
Bowman
D.M.J.S. & Harris S. “Conifers of Australia’s dry forests and open
woodlands. In: Ecology of the Southern Conifers pp252-270 eds. Enright NJ
& Hill RS. Smithsonian Institution Press, Washington DC.
Burfield
T (2000) Natural Aromatic Materials – Odours & Orgins pub AIA
Tampa.
2.
Sandalwood Update.
It
has been puzzling many of us exactly what is going on with Sandalwood oil EI –
suspicious as ever, some of us old hands suspect that some batches of oil are
being adulterated in new ways which we haven’t yet fathomed!
Sandalwood
Oil East African Osyris
lanceolata Hochst.
& Steud.
No
– not the Sandalwood East African material deriving from Tanzanian Osyris
tenuifolia Engl. (“bastard sandalwood”) which has lanceol as its
principle component! It was somewhat surprising for us to learn at this point
that there is a new kid on the block in the form of Sandalwood oil East
African from Osyris lanceolata. The scented wood from this
8-10m. tree native to S. Africa, makes an interesting oil, having an initial
strong sickly sweet note which rapidly gives way to a metallic-rubbery-woody
note slightly reminding of Cedarwood. The profile lacks the sensuality of E.I.
Sandalwood oil. The dry-own is a smooth somewhat sweet creamy woody note, much
less crude and more pleasant than the top note and more similar to E.I.
Sandalwood, but as noted for the top note, still lacking the sensual quality of
E.I. Sandalwood oil. Its highish concentration of santalols (probably 32% max)
and high santalyl acetate content (approx 35% typical) may make it seem an attractive proposition to some.
Apparently
150 tons of logs of Osyris lanceolata per month are being imported into
India by a company in Mumbai (which claims to be the largest importer) – and
who’s literature states sales of oils, chips, powder etc. are made into
chewing tobaccos, attars, perfumery and the agarbatti/joss-stick industries. The
company admits also to producing between 750-800 Kg of East African sandalwood
oil per month (Banker 2004). However the sustainability of this practice is far
from clear - reports of the threatened status of Osyris lanceolata in the
Eastern Arc mountains of Tanzania can be viewed at http://global.finland.fi/julkaisut/group_3.htm.
Further, a report on oil on the resource status of Osyris lanceolata in
Tanzania and oil quality variation amongst endemic trees populations by
Mwang'ingo, P.L. et al.can be viewed at http://www.inasp.info/ajol/journals/safj/vol199abs.html.
From these reports the situation would seem to show cause for concern.
Ref:
Banker R (2004) Personal communication to author.
Sandalwood
oil New Caledonian Santalum
austrocaledonicum Viell.
var. austrocaledonicum.
Now
being promoted and sold by several essential oil companies, it remains to be
seen how long this source, previously reported to be threatened, will last. So
what do we know?
We
know that sandalwood trees (Santalum austrocaledonicum) which grow from
5-12m. and may reach 30-45 cm. in girth, are widespread on the Isle of Pines and
in the Loyalty islands around Noumea and to the north of the main island. On
Grande Tierre it only occurs in a few restricted areas (SPRIG 2000). We also
know from the same source that three varieties are distinguished S. austrocaledonicum
var. austrocaledonicum, S. austrocaledonicum var. pilosulum,
S. austrocaledonicum var. minutum, and that morphological and oil
content differences occur between S. austrocaledonicum var. austrocaledonicum
trees on Loyalty Island and “the Ile des Pines” provenance. We also know
that subspecies of Santalum species might show some variations
sesquiterpenoid composition, however S. austrocaledonicum oils from
several geographic locations are known to be able to pass the ISO 3518 criteria
for Sandalwood oils, although the optical rotation criteria may be a stumbling
block.
New
Caledonia was reported as having 360,000 hectares of forest land but only 10,000
under cultivation ref: www.fao.org/DOCREP/004/Y1997E/y1997e19.htm
As the European
Forestry Institute points out at http://www.efi.fi/cis/english/creports/vanuatu.php
“In general, current timber export markets in Asia and New Caledonia do not
require information on the environmental standards and impacts of logging
operations”. This is important because energy intensive steam distillation of
small charges (250-300Kg) of sandalwood chippings or shavings to produce the
sandalwood crude oil (this crude grade is being sold into aromatherapy), take up
to 2 days to complete and thereby generate relatively large amounts of carbon
emissions per kilo of oil, contributing to the overall negative ecological
impact of the operation. As several minor Pacific Islands are currently being
submerged through the effects global warming, this is a sensitive issue. Cropwatch
has been making representation to Australian entrepreneurs in the Pacific
connected with Sandalwood exploitation suggesting that the implementation of
solar distillation rather than importing diesel to generate steam would perhaps
help reduce this negative impact, however it is to be remembered that Australia
is not a signatory to the Kyoto protocol and has little internal pressure to act
in a deep green ecological manner. It is also to be remembered that Oceana
itself causes a huge carbon emission loading to the world atmosphere which can
only be added to by diesel or wood-fired distillation processes.
Cherrier
(1993) reported on the difficulties of sandalwood cultivation in New Caledonia
noting heartwood development was proportional to proper development (fast
growing trees producing less heartwood). On the narrower subject of
sustainability, Ehrhart (1997) presented a fairly optimistic report on the
status of known consistent sandalwood stocks in New Caledonia (in contrast to
the depleted situation in many/most other South Pacific locations), and makes
the point that surveyed sustainable logging management should be possible in
these circumstances (yearly quotas have been set at 55 to 60 tons of wood).
However, apart from illegal cropping & fire damage, the danger is that of
over-exploitation – the bio-resources of New Caledonia to supply Sandalwood
oil are unlikely to be able to supply more than a few percent (i.e. probably no
more than 2 tons max.) of the total Sandalwood oil demand – which will be
severely tested now that leading French aroma houses are currently offering oil
from this origin. Further, as indicated above, whilst the emphasis in the sales
propaganda by Sandalwood oil salesmen has largely centered on examining tree
sustainability, the negative aspects concerning the total environmental impact
of the operation can easily be overlooked.
References:
Cherrier, J-F, 1993. “Sandalwood in New Caledonia”. In F.H. McKinnell (ed) Sandalwood in the Pacific Region. Proceedings of a symposium held on 2 June 1991 at the XVII Pacific Science Congress, Honolulu, Hawaii. Canberra: ACIAR Proceedings No.49. pp19-22.
Ehrhart
Y. (1997) “Descriptions of some Sandal Populations in the South West Pacific:
Consequences etc.” ACIR Proc. 84, 105-112.
SPRIG
(2000)
Santalum
album
plantations Australia
At the time of
going to press, a report about the lack of any impact assessment study ever
being carried out, and a statement concerning economic failure of investment
schemes for S. album plantations have had to be held over for a future
issue. Meanwhile mailed comments on the status of Santalum spp. in
Australia covered in Cropwatch 2 have been received by the author from two
senior Australian Forestry officials, who have unfortunately declined permission
to have their observations reproduced here.
3.
Tasmania: destruction of the forest eco-system.
Its hard to miss the press coverage on this
lately, with The Guardian reporting that concerns about Australian
forests are an election issue, and the singer Chrissie Hynde supporting the
boycott of Tasmania as a holiday destination etc. by People for the Ethnic
Treatment of Animals (Peta). Meanwhile loggers seem to prove once again prove
that whatever the country concerned, they are above the law and cannot be
stopped. Richard Flannagan (Guardian April 21, 2004 p16) wrote an
impassioned article about the setting alight of Tasmanian rainforest which is
felled before being napalmed, much of the wood being sold as unprocessed wood
chips. Magnificent Eucalyptus regnans trees of enormous stature and great
age are gone forever, and pictures of such a cleared area of the Styx Valley in
Tasmania have previously featured in an earlier Guardian feature by David
Fickling (Guardian March 22, 2004). Flannagan also describes the close
relationship that Tasmanian politicians enjoy with Gunns Ltd., the largest
logging company in Australia and how the population is cowed – to question
this action is to risk ostracisation or unemployment. Fickling mentions in more
detail that 2 board members of Gunns were criticised in an official bribery
inquiry in 1989, and the fact that Tasmania’s acting premier, Paul Lennon
visited Scandinavian pulp mills with Gunns chief executive, John Gray. Perhaps Cropwatch
is starting to understand why we meet a brick wall so many we try to communicate
with in that felled continent. Meanwhile comprehensive information on the
unsustainable activities of Gunns Ltd. can be viewed on the Wilderness Societies
website at http://www.wilderness.org.au/campaigns/corporate/gunns/
4. GM non-food
crops.
A very
well researched report by GeneWatch's director Dr. Susan Mayer at www.genewatch.org/CropsAndFood/Reports/non-food_crops_part2.pdf
identifies some research on GM crops intended for non-food use: grasses,
flowers, trees, and crops such as cotton used for fibre production. Tree species
referred to include Betula pendula, Eucalyptus camuldensis, Eucalyptus
globulus, Liquidamber spp. etc., and details of trials being
carried out in Canada on larches and black spruce figure amongst much other
identified work. Mayer notes that there are no GM trees available commercially
as such, but work has been carried out to transfer insect resistance, and
herbicide tolerance. In the flowers section of the report you will find
reference of "the molecular breeder" Florigene (offices in Australia
& Netherlands) and its’ interests in the cut flower industry – giving
relevant information on patents for roses, carnations, chrysanthemums. Mayer
also disturbingly reports on retailed mauve & violet GM carnations with
extended vase life sold by Florigene & Suntory in Australia & Japan
respectively.
In
India, Ashok Sharma reported in February this year writing in the Financial
Express (http://www.financialexpress.com/fe_full_story.php?content_id=53684)
that the Indian Minister for Agriculture Rajnath Singh inaugurated the Centre
for Transgenic Plant Development in Jamia Hamdard in Delhi. Sharma reports that
the centre has already developed a transgenic herb Chicory (Cichorium intybus
L.) which has a 40% higher content of esculin, which has skin protective
properties. Other ongoing work at the centre includes studies on ACC oxidase to
improve the shelf life of vegetables & fruits and chalcone synthase for
flower colour modulation. Since several Indian aromatic raw material producers
that the author has spoken to clearly understand that going down the GM route
would jeopardise sales of these materials into EU markets, these developments in
allied areas seem surprising.
5. OPINION:
Methyl eugenol-containing essential oils.
Copyright Tony Burfield May 2004.
Worries about possible risks due to the methyl
eugenol content of natural materials – herbs, essential oils - have surfaced
in the recent past but there is a dearth of information on the subject directly
available in the public domain to aromatherapists or complementary health
practitioners. The following feature is an attempt to add some background
information to this subject.
The warm, musty-mild-spicy odoured aromatic
compound Methyl Eugenol (aka eugenol methyl ether, or
4-allyl-1,2-diomethoxybenzene) is
prohibited from being directly added as an ingredient to fragrances intended for
retailed cosmetic products, due to worries about its’ potential
carcinogenicity.
As it occurs naturally in many essential oils and extracts, the addition of
these ingredients is not restricted outright, but on provision that the methyl
eugenol content does not exceed the following concentration in the following
finished products according to the IFRA standards (see www.ifraorg.org/):
Fine Fragrances
0.020%*
Eau de Toilette
0.008%
Fragrance Cream
0.004%
Rinse off products
0.001%
Leave-on products/
Oral hygiene products 0.0004%
Non skin (as defined on IFRA website)
0.010%*.
*The
limit of 0.02% for the starred items applies to the concentration in the
fragrance compound.
In effect this means that there is an obligation
on ingredient suppliers, under the requirements of due diligence, to supply
information to customers, to
make sure that they receive the necessary information in order for them to
comply with the above requirements of the IFRA Standards. To spell this out in
more detail, reporting the methyl eugenol content of the specific batch of the
ingredient will then allow the customer to further calculate final levels of
methyl eugenol appearing in the finished product. It is difficult to see how
many small essential oil suppliers, without resort to internal analytical
expertise, will be able to perform this function. Additionally, it is relatively
easy to find plants for sale on the Internet, who’s essential oils contain
high levels of methyl eugnol e.g. Black tea tree plants can be ordered at http://www.hotkey.net.au/~macs_oils/plant01.htm.
No warning about the potential toxicity of methyl eugenol is presented.
It has long been established that methyl eugenol
occurs in essential oils such as Canadian Snake root, Bay, Citronella, Laurel,
Emodia, Fennel, Betel, “Brisbane Sassafras”, Pimento, Hyacinth etc., and its
occurrence often coincides with the additional presence of eugenol (Poucher
1991). And so, purely as a guide, here below is presented a “snapshot” guide
to the reported methyl eugenol content of several further essential oils.
Published data on Methyl Eugenol Contents of Essential Oils.
1.
FEMA have published data to members on methyl eugenol contents of
essential oils (no geographic origins specified).
2.
The BFA on 12.02.02 circulated BEOA data from 09.11.01 on the
methyl eugenol content of a number of analysed commercial oils. Oils were
classified by botanical name (no chemotypes were distinguished) and by origin.
There are no particular surprises, although methyl eugenol contents on
rose otto seemed low-ish compared with other published data, and the range of
methyl eugenol contents of the 23 basil oils (all apparently from Egypt) was
relatively large. No data on fennel oil (identified by the EU Scientific
Committee on Food as a dietary source of methyl eugenol) was included.
The BEOA data document makes comment that expert analysis of genuine
essential oils shows how widely essential oils vary in composition, and makes
comment that the BACIS commercial data-base of essential oils shows methyl
eugenol contents of 258 oils, that some of this data is misleading, and not
representative of genuine high volume essential oils used in commerce.
3.
IFRA data on methyl eugenol contents of essential oils, as
presented on the IFRA website www.ifraorg.org
in May 2004 does not define the plant source species, the geographical origins
of oils or any chemotype information. A document circulated by IFRA (to members
only – not in the public domain – but most of the information the same as on
the IFRA website) on April 6th 2004 lists 21 essential oils, again
giving no botanical identification, only giving geographic origins for two types
of oils (citronella and rose), and giving chemotype information for basil only.
As has been observed previously by this author, the standard of botanical
reporting in IFRA documents, and in EU legislation leaves a great deal to be
desired.
4.
A list of plants containing methyl eugenol, duplicating the
species names of many of the entries below, can be found on the Agricultural
Research Services data-base
at http://www.ars-grin.gov:8080/npgspub/xsql/duke/chemdisp.xsql?chemical=METHYL-EUGENOL
Table I - Various References re:
Methyl Eugenol content of EO’s.
| Essential oil | Remarks | Methyl
eugenol content |
Reference key (see below) |
| Acorus
calamus |
Calamus Indian |
1.0% |
Shiva
et al. |
| Acorus
calamus |
Calamus
Mediterranean |
0.9%
max |
BEOA |
| Acorus
calamus (?) |
Calamus oil |
<1.0% |
IFRA website IFRA 06.04.04 |
| Anasarum
canadense |
Snakeroot oil |
36.0-
45.0% |
EOS |
| Aniba
rosaedora |
Rosewood oil |
0.11% |
TQ |
| Artemisia
dracuncunculus |
Tarragon
oil Russian type |
11.5%
|
TB |
| Artemisia
dracuncunculus |
Tarragon
oil Russian type
|
5 – 29% |
EOS |
| Artemisia
dracuncunculus |
Tarragon
oil French type |
0.8% |
TB |
| Artemisia
dracuncunculus |
Tarragon
oil French type |
0.1 to 1.5% |
EOS |
| Artemisia
dracuncunculus (?) |
Estragon
oil |
<1.5% |
IFRA
website IFRA 06.04.04 |
| Canarium
indicum |
Essential
oil |
300-750
ppm |
Duke 2 |
| Canarium
lucozonium |
Elemi
oil Philipines |
0.44% |
TQ |
| Cananga
odorata
subsp. macrophylla |
Cananga
oil |
0.17% max |
BEOA |
| Cananga
odorata subsp. macrophylla
(?) |
Cananga
oil |
<0.5% |
IFRA
website IFRA 06.04.04 |
| Cananga
odorata subsp.
genuina |
Ylang
ylang IInd
quality |
0.15% |
TB |
| Cananga
odorata
subsp. genuina |
Ylang
ylang. No details. |
0.154% |
TQ |
| Croton
elutaria |
Cascarilla
oil W.I. |
0.2% max |
BEOA |
| Croton
elutaria (?) |
Cascarilla
oil W.I. |
<1.0% |
IFRA
website IFRA 06.04.04 |
| Cinnamomum
camphora |
Camphor
oil white, China |
Not detected |
BEOA |
| Cinnamomum
cassia |
Cassia
bark oil China |
0.03% max. |
BEOA |
| Cinnamomum
cassia (?) |
Cassia
oil |
<0.1% |
IFRA
website IFRA 06.04.04 |
| Cinnamomum
tamala |
Tejpat
oil |
0.5% |
Lawr |
| Citrus
paradisi |
Grapefruit
oil |
0.0002% |
TQ |
| Citrus
sinensis |
Sweet?
orange oil |
0.0004% |
TQ |
| Cymbopogon
citratus
|
geraniol
chemotype |
to 18.0% |
TB |
| Cymbopogon
nardus |
Sri
Lanka |
1.8% max. |
BEOA |
| Cympopogon
nardus |
Sri
Lanka |
3.0% |
FEMA |
| Cymbopogon
nardus (?) |
Citronella
oil Sri Lanka |
<0.2% |
IFRA 06.04.04 |
| Cymbopogon
winterianus |
Citronella
oil, China (Java type) |
0.2% max. |
BEOA |
| Cymbopogon
sp. |
Citronella
oil |
<2.0% |
IFRA
website |
| Cymbopogon
winterianus
(?) |
Citronella
oil Java |
<2.0% |
IFRA 06.04.04 |
| Dacrydium
franklinii |
Huon
Pine Oil |
to 98.0% |
TB |
| Daucus
carota |
Carrot
seed oil |
0.165% |
TQ |
| Daucus
carota |
Carrot
seed oil Chinese |
1.23% |
Kam |
| Daucus
carota |
Carrot
oil |
<0.5% |
IFRA
website IFRA 06.04.04 |
| Daucus
carota |
Carrot
oil CO2 extract |
0.1% |
IFRA |
| Echinophora
tenuifolia
|
Turkey |
17.5
– 50.0% |
TB |
| Elettaria
cardamomum |
Cardamom
oil, India |
tr. to 0.1% |
TB |
| Eucalyptus
(globulus?) |
sp. name
not indicated |
1.07% |
TQ |
| Hyssop
|
sp. name
not indicated |
0.55% |
TQ |
| Hyssopus
officinalis (?) |
Hyssop
oil |
<1.0% |
IFRA
website IFRA 06.04.04 |
| Illicium
verum |
Star
Anise oil |
0.11% |
TQ |
| Laurus nobilis | Bay
Laurel oil |
2.8% max. |
BEOA |
| Laurus
nobilis
|
Bay
Laurel oil |
4.0% |
TB |
| Laurus
nobilis
|
Bay
Laurel oil |
4.62% |
TQ |
| Levisticum
officianale |
Lovage
Leaf |
1.3% max. |
BEOA |
| Levisticum
officianale (?) |
Lovage
leaf oil |
<1.5% |
IFRA
website IFRA 06.04.04 |
| Lippia
citriodora
|
Verbena
oil |
2.3% |
TB |
| “Magnolia”
|
Michaelia
or Magnolia spp. ?? |
2.64% |
TQ |
| Melaleuca
alternifolia |
Tea tree
oil |
trace |
IS |
| Melaleuca
bracteata |
(chemotypes
II, III, IV) |
to >40% |
TB |
| Melaleuca
bracteata |
(chemotypes
I,II,III, IV) |
trace;
1.5%; 8.7% and 50% respectively |
Brophy et al. |
| Melaleuca
leucadendron
|
(chemotype
II, methyl eugenol form) |
95-97% |
TB |
| Melaleuca
leucadendron
|
(chemotype
I, Ila and llb) |
1.6,
94.6 and 6.7% respectively |
Brophy JJ |
| Michelia
alba |
Flower
and leaf oils |
0.38
& 0.22% respectively |
Kam. |
| Myrstica
fragrans |
Nutmeg
Oil Sri Lanka |
0.8% |
TB |
| Myrstica
fragrans |
East
Indian Nutmeg oil |
tr – 1.2% |
EOS |
| Myrstica
fragrans |
West
Indian Nutmeg oil |
0.1- 0.2% |
EOS |
| Myrstica
fragrans (?)
|
Nutmeg
oil |
<
1.0% |
IFRA
website IFRA 06.04.04 |
| Myrstica
fragrans (?)
|
Mace oil |
<
0.5% |
IFRA
website IFRA 06.04.04 |
| Myrtus
communis |
Myrtle
oil |
1.21% |
TQ |
| Myrtus
communis |
Myrtle
berry oil |
2.3% |
Mazza |
| Ocimum
basilicum |
Sweet
basil oil |
Often
below 0.2%,
Comores (exotic
type) to 1.6% |
|
| Ocimum
basilicum |
Oil of
Egyptian origin |
5.6%
max |
BEOA |
| Ocimum
spp. |
Basil
oil |
<
6.0% |
IFRA
website IFRA 06.04.04 |
| Ocimum
basilicum |
Basil
Oil |
2.6%
|
FEMA |
| Ocimum
basilicum var.
basilicum |
Described
by F & P as Exotic type Basil oil |
1.6% |
F & P. |
| Ocimum
basilicum var. “feuilles
de laitre” |
Described
by F & P. as European type Basil oil |
2.5
to 7% |
F & P. |
| Ocimum
basilicum var. “grand
vert” |
Oil |
55-65% |
F & P. |
| Ocimum
basilicum var.
minimum |
Described
by F & P. as “Small Basil” |
55-65% |
F & P. |
| Ocimum
gratissimum var.
thymoliferum |
Described
by F & P. as “Basil oil thymol type” |
1.7% |
F & P. |
| Ocotea
pretiosa |
(Brazilian
Sassafras oil- methyl eugenol type)
|
> 50.0% |
TB |
| Pelargonium
graveolens |
Geranium
oil China Geranium oil Bourbon |
Not
detected in either
oil |
BEOA |
| Pelargonium odoratissum |
Geranium
oil Egypt |
Not
detected |
BEOA |
| Peumus
boldus |
Leaf |
100-125
ppm |
Duke |
| Pimenta
dioica |
Pimento
leaf oil |
to
2% |
TB |
| Pimenta
dioica |
Pimento
leaf oil |
2% |
FEMA |
| Pimenta
dioica |
Pimento
leaf oil |
15.4% |
TQ |
| Pimenta
dioica |
Pimento
leaf oil |
3.9% |
BEOA |
| Pimenta
dioica |
Pimento
berry oil |
to
8% |
TB |
| Pimenta
dioica |
Pimento
berry oil |
15.0% |
BEOA |
| Pimenta
dioica (?) |
Pimento
berry oil Pimento
leaf oil |
<
15.0% <15.0% |
IFRA
website IFRA 06.04.04 |
| Pimenta
dioica |
Plant
part to produce oil not stated |
1.2 –
4.4% |
F
& P. |
| Pimenta
racemosa var.
racemosa |
Methyl
chavicol/methyl eugenol chemotype |
48.1% |
Aurore et al. |
| Pimenta
racemosa |
Bay
leaf oil |
4.6%
|
TQ |
| Pimenta
racemosa |
Bay
leaf oil
|
0.4
to 12.6% |
TB |
| Pimenta
racemosa (?) |
Bay
oil |
<
4.0% |
IFRA
website IFRA 06.04.04 |
| Pimpinella
anisum |
Anise
oil |
0.11% |
TQ |
| Piper
cubeba |
Cubeb
oil |
Not
detected |
BEOA |
| Ravensara
aromatica |
Ravensara
oil Madagascar |
0.10% |
F. & P. |
| Rosa
centifolia
|
Rose
absolute |
0.6%
to 1.9% |
TB |
| Rosa
centifolia
|
Rose
otto |
1.1
to 3.0% |
TB |
| Rosa
damascena
|
Rose
otto |
1.1
to 3.0% |
TB |
| Rosa
damascena
|
Rose
otto Bulgaria |
1.6%
max |
BEOA |
| Rosa
spp. |
Rose
oil Bulgaria “different types” |
<
2.5% |
IFRA 06.04.04
|
| Rosa
sp. |
Rose
oil China |
<
3.5% |
IFRA 06.04.04 |
| Rosa
damascena
|
Rose
otto Morocco |
0.5%
max |
BEOA |
| Rosa
sp. |
Rose
oil Morocco |
<2.6% |
IFRA 06.04.04 |
| Rosa
damascena
|
Rose
otto Turkey |
0.5%
max |
BEOA |
| Rosa
sp. |
Rose
oil Turkey |
<3.0% |
IFRA 06.04.04 |
| Rosa
sp. |
Rose
oil |
<3.5%
|
IFRA
website |
| Rosa
damascena
|
Absolute |
0.8
to 1.6% |
TB |
| Rosa
damascena
|
Rose
otto India |
2.0-2.5% |
Shiva et al. |
| Rosa
spp. |
Rose
bud oil Georgia |
<0.1% |
TBb |
| Rosa
rugosa |
Rose
otto, China |
0.10% |
SCIB |
| Rosmarinus
officinalis |
Rosemary
oil |
0.011% |
TQ |
| Rosmarinus
officinalis |
Rosemary
oil Tunis |
>0.01% |
TBa |
| Satureia
hortensis |
Summer
savoury oil |
0.88% |
TQ |
| Satureia
montana |
Winter
savoury oil |
0.11% |
TQ |
| Satureia
montana |
Winter
savoury oil Balkans |
0.7% |
BEOA |
| Satureia
montana (?) |
Winter
savoury oil |
<1.0% |
IFRA
website IFRA 06.04.04 |
| Syzygium
aromaticum |
Clove
bud oil |
to
0.15% |
TB |
| Syzygium
aromaticum |
Clove
bud oil |
0.2% |
Shiva et al. |
| Syzygium
aromaticum |
Clove
leaf oil Indonesia |
0.5%
|
TB |
| Syzygium
aromaticum |
Clove
oil |
<0.5% |
IFRA
website IFRA 06.04.04 |
| Tagetes
minuta |
Tagete
oil |
0.03% |
Lawr. a |
| Trachyspermum
ammi |
Ajowan
oil, India |
0.03% |
TBb |
N.B. Question marks in the above
table appear when the author has had to make an intelligent guess at the
botanical origin of the oil because the original source failed to reveal it.
Remarks on the Toxicity of
Methyl Eugenol.
Similarities
of methyl eugenol to the structure of safrole, a known carcinogen, have not gone
un-noticed.
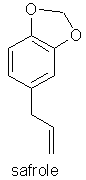
Following
the RIFM/FEMA workshop in May 2000, the FEMA expert panel issued a report
entitled “Safety Assessment of Allylalkoxybenzene Derivatves Used as
Flavouring Substances – Methyl Eugenol and Estragole”. This included a
description of the 2-year bioassay with methyl eugenol by the National Toxicity
Program (NTP) whose aim was to establish the carcinogenic potential of methyl
eugenol regardless of route of administration. The NTP study found that the
present exposure to methyl eugenol from food (mainly intentional addition of
essential oils, spices and spice isolates) presents
no hazard to human health. The report finds that although very high doses
are carcinogenic, they are such that they must have first induced a hepatotoxic
effect. We also subsequently
learned from the RIFM/FEMA workshop write-up, that one serving of pesto contains
from 10-100 times the average daily human consumption of methyl eugenol,
although even this level was 100 times lower than the lowest dose forcibly given
to animals in the NTP assay.
The NTP technical
study on the 2 year toxicology and carcinogenisis studies on methyl eugenol in
F334/N rats and B6C3F1 mice was published in July 2000. It showed clear
evidence of carcinogenic activity of methyl eugenol in the tested rodents,
and can be viewed at http://ehis.niehs.nih.gov/ntp/docs/tr491/tr491abs.pdf
The German
Bundesrat decided on May 11th 2001 not to market flavourings and
foodstuffs containing added methyl eugenol (or methyl chavicol) after June 30th
2001, although this ruling did not apply to methyl eugenol naturally present in
flavourings or foodstuffs.
The EU’s
Scientific Committee on Food expressed an opinion on methyl eugenol on 26.09.01,
which can be viewed at http://europa.eu.int/comm/food/fs/sc/scf/out102_en.pdf
The committee remarked that methyl eugenol is a multi-site, multi-species
carcinogen, being both genotoxic and carcinogenic. Average
human intake from diet of methyl eugenol amounted to 13 mg/person/day and the
97.5th
percentile
was 36 mg/person/day (on a body weight basis these values correspond to 0.19 and
0.53 mg/kg bw/day, respectively). The
committee was unable to establish a safe exposure limit.
Subsequently IFRA
decided to severely restrict the limits of methyl eugenol in finished fragranced
products in 2001 (36th Amendment to the Code of Practice).
Low methyl eugenol rose oil has been
commercially offered by a small number of aroma houses. Removal of the methyl
eugenol content by high vacuum fractional distillation seems to adversely affect
the typical rose character in products offered. Removal of the methyl eugenol
content by spinning band or spinning cone distillation may be more satisfactory,
but production time is at a premium on this expensive technology. Rose oils
naturally very low in methyl eugenol are known in Eastern Europe and further
East, but the quality is very poor to actually unacceptable for most purposes,
even before methyl eugenol removal.
As a closing comment, it is hard to see why
the aromatherapy and cosmetic industries are “led by the nose” on the choice
of available commercial rose qualities utilised, which merely reflect historical
perfumery trade uses. It has previously been established that rose absolutes
from varieties of garden roses can demonstrate beneficial cosmetic properties (Étienne
et al. 2000) whereas a conventional commercial rose absolute showed none of
these effects. Further, it is likely that certain of these other varieties will
only present a fraction of the methyl eugenol levels encountered in
conventionally sourced rose ottos and absolutes.
Methyl
Eugenol in Aromatherapy.
The author is
unable to find any detailed advice given by professional
aromatherapy organisations to members on this issue, on a par with that put by
IFRA for its membership in the perfumery profession. Harris (2002) has
reviewed the position of methyl eugenol in aromatherapy practice in the light of
IFRA restrictions in the fragrance industry. It is worth exploring a number of
points.
Firstly, Harris
notes that the IFRA have published a list of essential oils (e.o.’s) with
methyl eugenol contents, commenting that these figures only pertain to oils used
in the fragrance industry. Harris instead quotes e.o. data from Lawrence
(1998-2002). However IFEAT have previously criticised the use of Lawrence’s
data (specifically over the separate 26 allergens issue), as they maintain it is
relates only to experimental data and does not relate to the composition of
commercial oils. In any case, in the real world, the e.o.’s distributed
by many (but certainly not all) aromatherapy oil suppliers are identical to
those distributed by the fragrance industry.
Harris
further maintains that “the average aromatherapy treatment regime consists of
5-10 sessions, given at most once per week, generally with the essential oils
employed being changed during this regime according to the improvement of the
client…”, and goes on to state, “those most at risk from methyl eugenol
are the aromatherapists themselves”, but does not investigate exposure of this
most “at risk” group in any satisfactory detail. Harris further mentions
avoidance of high methyl eugenol containing oils by therapists, and talks of
using “3 drops in a blend” – which, as several professional therapists
have privately commented, “is not
Since
aromatherapy is a poorly paid profession, many professional aromatherapists are
obliged to work extended hours, and may have to perform 6-8 massages per day,
5-6 (or more) days per week. Further, a whole body massage may well be carried
out with 20-50 mls of massage oil containing 2-2.5% e.o., although some
practitioners apparently have been known to use even higher concentrations (Guba
1998). Unknown amounts of methyl eugenol are therefore absorbed by the therapist
throughout the week, via skin absorption through the hands, and by inhalation of
vapour. Harris doesn’t mention the fact that diet is additionally adding to
the therapist’s body burden of methyl eugenol.
The
above factors may eventually allow a more realistic calculation of daily human
body loading from methyl eugenol for aromatherapists, but interpretation of the
data revolves around interpretations of the NOEL (no-effects) level in the
longer-term and appropriate safety factors (IFRA used a factor of 1000 X).
Since aromatherapeutic treatments such as whole body massage are vastly
different from animal dosing studies, drawing direct conclusions about possible
toxicological effects is distinctly risky. Further, it is already known from
human liver microsomal preparations that metabolism rates by human cytochrome
P450 isozymes for methyl eugenol varies more than 37-fold (Gardner et al. 1997)
suggesting a wide range of serum concentrations will occur in the general
population following methyl eugenol exposure.
Meanwhile
Schecter et al. (2004) have produced a study on human consumption of methyl
eugenol and its elimination from serum under a mandate from the National
Toxicology Program of the US Department of Health and Human Services. In
particular the team investigated the consumption of methyl eugenol from a brand
of gingersnaps, found to contain a relatively high concentration of methyl
eugenol at 3.3mg/g
(a number of other foodstuffs containing lower concentrations of methyl eugenol
are also listed in the article & cigarette tobacco’s were identified as
another possible source of methyl eugenol exposure!). Serum peak levels of
methyl eugenol were found to be within range of a concurrent study of 213
non-fasting subjects in the third Nutrition Examination Survey (NHANES III,
1988-1994). However in this latter study, the authors found that methyl eugenol
levels in the blood of the general US population were higher than expected (but
the highest concentration found, 390pg/g, was still 2000 X lower than the lowest
dose used in the NTP rodent studies referred to above). Nevertheless, as
Schecter et al. remark, the significance of the elevated levels with respect to
any toxicological consequences, still remains to be determined.
It
may well eventually turn out that a working aromatherapist, constantly using
basil and rose oils, and with a fondness for pesta and flavoured cigarettes is
more likely to be hit by a meteorite than to contract a toxicological problem
due to daily methyl eugenol exposure from all these routes. Its just that it
would be nice to think that those entrusted with a duty of care towards working
people in our society were actively investigating this topic. The situation
being as it is, assessments on this topic are more likely to be made by
self-educated laymen, than by formerly qualified toxicologists – and to this
end, Cropwatch has written to some toxicologists for some learned
opinions on this matter. Any replies will be published in further editions of
this organ.
STOP
PRESS!
Professor
Arnold Schecter (see reference above) kindly read my piece on methyl eugenol
above and hinted from the tone of the article above that I might have
understated the risk slightly, commenting further as follows:
“What my work
followed during a year I worked at NIH was that methyl eugenol is extremely
carcinogenic to rodents and causes cancers in rats and mice, two species, and in
multiple tissues. The human levels may or may not be of concern, both those we
reported and the higher levels we alluded to in the general US population, so
high for unknown reasons. ME does not occur by itself in humans but in
combination with many other toxic chemicals so potential human health effects
might be from ME alone or in combination with others”.
And further: “In
combination with other carcinogens (methyl eugenol) might be harmful at
lower levels than those derived dosing animals with one chemical only. Many
chemicals in our bodies.”
I
take these points on board, and suggest even louder now, that the aromatherapy
profession needs to take this issue seriously, perhaps appealing for outside
help to more properly evaluate the risk.
Glossary
BFA:
British Fragrance Association
IFEAT:
International Federation of Essential Oils and Aroma Trades
IFRA:
International Fragrance Research Association
Table Data References:
Aurore, G. S. Abaul, J. Bourgeois, P. Luc, J. (1998) “Antibacterial and Antifungal Activities of the Essential Oils of
Pimenta racemosa var. racemosa P. Miller (J.W. Moore) (Myrtaceae).”
J. Essential Oil Res. 10(2),
161-164.
BEOA:
British Essential Oils Association 9th Nov 2001 – data reproduced
by kind permission.
Brophy JJ: Brophy JJ (1999)
“Potentially Commercial Melaleucas” in Tea Tree – the Genus Melaleuca
eds. Ian Southwell & Robert Lowe. Harwood Academic Publishers.
Brophy et al: Brophy et al. (1999) J
Essen Oil Rec 11, 327-332.
Duke: Duke J (?) from Chemicals
and their Biological Activities in: Peumus boldus MOLINA (Monimiaceae)
– Boldo – see http://www.rain-tree.com/db/Peumus-boldus-phytochem.htm
Duke 2 : see
http://www.ars-grin.gov:8080/npgspub/xsql/duke/chemdisp.xsql?chemical=METHYL-EUGENO
EOS:
“Essential Oil Safety” Robert Tisserand & Tony Balacs
Churchill-Livingstone 1996.
F & P: Franchomme P. &
Peneol D (1995) “l’Aromatherapie
Exactement” pub. Jollois. R.
Guba R (1998) “Toxicity Myths
–the Actual Risks of Essential Oil Use.” Centre for Aromatic Medicine 1998.
IFRA website: www.ifraorg.org
information as at 01.05.2004
IFRA: Annex 1 IFRA Standards.doc April 6, 2004.
IS: Ian Southwell (1999) “Tea
Tree constituents” in Tea Tree – the Genus Melaleuca eds. Ian Southwell
& Robert Lowe. Harwood Academic Publishers
Kam: Kameoka H. (1993) “The
Essential Oil Constituents of Some Useful Plants from China” in Recent
Developments in Flavour & Fragrance Chemistry –Proceedings of the 3rd
Int. Haarman & Reimer Symposium Pub. VCH NY 1993.
Lawr.: Lawrence BW (1989) EO’s 1981-7 Allured Publ.
Lawr. a: Lawrence BM et al. (1985) Perf
& Flav 10(6), 56-58 Dec 1985-Jan 1986
Mazza G. (1983) “GCMS
Investigation of Volatile Components of Myrtle Berries” J. Chromatog. 264,
304-311.
SCIB: Zhu Lianfeng et al. (1993) Aromatic
Plants & Essential Constituents South China Inst of Botany, Hai Feng
Publishing Co.
Shiva et al: Shiva MP, Lehri A,
Shiva A. (2000) Aromatic & Medicinal Plants pub IBD 2000.
TB: Tony Burfield (2000) Natural
Aromatic Materials: Odours and Origins pub. AIA Tampa.
TB see: http://www.users.globalnet.co.uk/~nodice/new/magazine/odprofile.htm
TBb: Tony Burfield (unpublished
data)
TBc: Tony Burfield & Sylla
Sheppard-Hanger (2002) “Basil Oils Monograph” AIA UK 2002.
TQ: trade suppliers questionnaire (IFF
2003)
Poucher (1991) Poucher’s
Perfumes, Cosmetics and Soaps - Vol 1 The Raw Materials of Perfumery 9th
edn. Blackie Academic & Professional.
Zhu Liangfu et al. (1993) Aromatic
Plants & Essential Constituents South China Inst of Botany.
Text
References.
Étienne et al. (2000) “New and
unexpected cosmetic properties of perfumes. Effects upon free radicals and
enzymes induced by essential oils, absolutes and fragrant compounds.” International
Journal of Cosmetic Science 22, 317-328.
Gardner et al. (1997) “Cytochrome
P450 mediated bioactivation of methyleugenol in Fisher 344 rar and human liver
microsomes.” Carcinogenesis 18, 1775-1783.
Harris
B. (2002) “Methyl eugenol – the current bete noir of aromatherapy”. Int.
J. of Aromatherapy 12(4),
193-201.
Lawrence
B.W. Progress in Essential Oils (1998-2002).
NHANES III 1988-94 National Centre for Health Statistics (1994). Plan and
Operation of the Third National Health & Nutrition Examination Survey,
1988-94. Series 1: Program & Collection Procedure No 32.
Schecter A et al. (2004) “Human
Consumption of Methyleugenol and Its Elimination from Serum” Environmental
Health Perspectives 112(6), 678-680.