
The
Odour Perception of Essential Oils.
Copyright Tony
Burfield Jan 2003
(This article first appeared in Aromatherapy Today Vol 25, March 2003, pp33-37, and appears here by kind permission of the editor, John Kerr).
Introduction.
Some basic principles of the odour profiling of essential oils were set out in a previous article (Burfield 2002), the entire text of which is displayed on the internet, at: http://www.users.globalnet.co.uk/~nodice/ in the magazine section. This further article briefly explores new topics around the subject, including a little more on the differences between the scents given off by living plants vs. the odour profiles of essential oils and absolutes, some brief examples in odour classification, and the deleterious effects of adulteration on odour properties of essential oils.
Living Odours of Plants.
A few parts of this planet are heavily suffused with the aromatic vapours of odourous plants and have given travellers intense emotional feelings from interaction of aerial scents with their nervous systems. King J.R. (1998) - a consultant psychiatrist -comments that the herb-scented mountain air of the Malvern Hills (UK) should be prescribable - this pales somewhat against the heavy aroma of the Marquis, an area of aromatic plants growing on the littoral borders of some parts of the Mediterranean! Further, the effects of the profusion of alpine herbs growing in Himalayan mountainsides have been sufficient to give individual trekkers (and early botanists alike!) deep emotional experiences, which might have been based in reminiscence or the release of emotion. This is surely a perfect working example of aromatherapy in its very purest sense. In England, in times past, the heavy floral odour of blossom has apparently been enough to cause young maidens to swoon.
But not all flowering plants are associated with pleasant odours. In Europe, yellow fields of flowering mustard-seed rape emit irritating substances including a number of alkyl and aromatic nitriles & isocyanates, much to the annoyance and suffering of people living in close proximity, who have suffered respiratory problems both from the pollen and from the irritant volatile chemicals. In the Himalayas, scents from two plants are reported as frequently causing headache and vomiting. (Pandey G. 2000). These are Rhododendron anthropogon (from which Anthropogon oil is produced, used in perfumery to a minor extent) and the threatened plant Saussurea lappa (from which Costus oil and absolute are made and illegally traded). More familiar to aromatherapists, perhaps, is Wormseed oil from Chenopodium ambrosioides reported as liable to cause headache in persons inhaling the oil even briefly (Tisserand R. 1985).
The odours of fresh flowers and leaves, however, often differ markedly from the odour of the absolutes and essential oils made from them, and now with the availability of modern instrumental techniques like Mass Spectroscopy, we are able to capture and analyse the volatiles of the living plant and compare them with those extracted from dead vegetation. Kaiser R. (1997) for example describes how the air-borne odours from herbaceous plants in the low marquis area of the Ligurian coast, such as Thymus vulgaris, Euphorbia spinosa, Helichrysum italicum, Lavandula stoechas, and Pistachia lenticus, Spartium juneceum etc. in the middle marquis may be analysed, and gives headspace chromatograms for Maritime Pine (Pinus pinaster) resin exposed to sunshine, and Pistacia terebinthus amongst others. Amazingly, under these conditions, resin from Pinus pinaster yields several musky compounds, such as 13-tetradecanolide, 15-hexadecanolide & 16-hexadecanolide which are the secret of its sweet fragrant character, blown into the marquis from nearby woods containing these trees:

No one can argue however with the convenience of a bottleful of essential oil, which is more transportable and more seasonally available than rarefied mountainside air full of fragrant herbs! But many would regard that the aromatic extracts from a solvent extraction process give a “truer to nature” impression than the essential oils produced via a steam distillation process, since lower temperatures are used, leading to fewer artifacts and less loss of fragile, and/or low boiling components.
To interpret and classify odours, and to talk a descriptive odour language that other workers and colleagues can understand, needs teamwork and a pre-defined set of standards. According to polls, most people instinctively associate the term “floral” for example, with the odour of rose, but the employment of complex and multi-faceted natural odours as standards (like the odour of rose blossoms) has been criticised. The concept of a “green odour” can be best standardised against a single odourant: cis-3-hexenol, which can be smelled in freshly cut grass. This intense fresh green grass-smelling odourant derives from transformation of trans-2-hexenal, one of the first breakdown products of oleic acid:
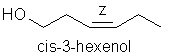
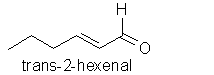
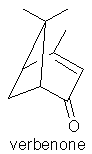
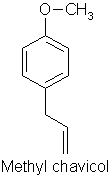
Similarly, a picked rosemary herb contains cis-3-hexenol, trans-2-hexenol, and hexenol, but these components are not present in the headspace of the fresh herb itself. Even more surprising is the fact that eucalyptol and camphor according to Mookherjee (1989) are only present in low concentrations in volatiles from the living herb (0.7-2% and 0.2% concentrations respectively), in contrast to their relative abundance in the essential oil. Whereas some workers have regarded verbenone as being an important character impact compound in rosemary essential oil, Lamparsky (1987) quoted cis- and trans-ocimenes, calamnene and calcorene as contributing to the typical odour characteristics of the oil. In contrast, and perhaps unexpectedly, the authentic odour of the fresh herb owes much to minor amounts of methyl chavicol (in conjunction with the odour of the other constituents) for its characteristic odour.
Finally, much poor olfactory quality Rosemary oil is produced, and may be unsaleable - it may have high a-pinene, and low borneol and 1,8-cineol contents which detract from its requirement to be fresh, cineolic, herbal-agrestic, camphoraceous and maybe a little woody. It is probable that the composition of rosemary oil is partially reflective of the depletion of water-soluble components during distillation, whilst adulteration accounts may often account for quality loss in other respects. Shortages from higher demand, and droughts in N. African districts have particularly exacerbated worries about the authentication of many traded oil stocks recently. Many buyers additionally demand very low iso-borneol and iso-bornyl acetate contents for Rosemary oil qualities, taking higher values as a primary rough indication of adulteration by a synthetic mix which typically might include borneol, camphor and isobornyl acetate, amongst others.
Odour Classification Schemes.
Many schemes have been devised to classify odours in perfumery. Those based
on the familiarity of natural products would seem appropriate for
Aromatherapists and Natural Perfumers, and some of the classification categories
such as citrus, woody, herbal, spicy, balsamic etc. are easily comprehended (Burfield
2002a). Some workers have sought to explain odour profiles and oil composition
in causative terms, for example Morales R. (1986) looking at aromatic herbs
particularly thinking perhaps of Thymus spp., remarked that generally
phenols occur when plants grow at high
temperatures in a dry climate, linalol and a-terpineol
increase in plants growing under humid conditions, and the geraniol content is
higher in a wet and cold climate.
Some workers attempt more sophisticated classifications
within odour categories: Kaiser R. (1993) has produced a classification scheme
for floral odours, dividing up on the basis of olfactory and chemical
criteria. He describes four types of flower scent:
Just looking at the “white floral” image group for now, the constituents linalol, nerolidol/farnesol, and aromatic esters are held in common, but the following differences in scent composition are important giving rise to the recognition of the scents of:
Jasmine sambac (Jasminum sambac) benzyl acetate, indole, methyl anthranilate, jasmone, methyl jasmonate
Gardenia (Gardenia spp.) tiglates of cis-3-hexenol, benzyl alcohol, jasmin lactone, other lactones
Honeysuckle (Lonicera spp.) cis-3-hexenol, jasmone, methyl jasmonate, indole
Orange flower (Citrus aurantia) indole, methyl anthranilate, 1-nitro2-phenylethane, phenacetaldoxime.
You will note the importance of nitrogen compounds to the floral aromas above - three of which are illustrated below. The importance of nitrogen and sulphur compounds in essential oils and natural raw materials seems to be a subject that aromatherapy teachers and aromatherapy oil analysts alike seem to be guilty of virtually ignoring.
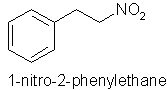
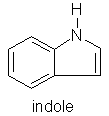
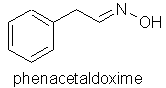
The rosy-floral, and ionone-floral image groups of floral perfumes above are
classified chemically in similar fashion; but the spicy-floral group only
consists of one example - that of carnation - where spiciness is afforded by the
eugenol content. Other examples of spicy-floral odours might be suggested –
Wallflowers (Cheiranthus
cheiri)
so typical of Victorian cottage gardens, and Helichrysum oils from various Helichrysum
spp. immediately spring to mind.
Periodicy in Fragrance Release.
Scent release in flowers is controlled by an internal biological clock, so that not only the concentration of fragrance often varies over the day, but so too does its composition, such that it will attract insect pollinators at the appropriate time of day – Jasmine sambac for example is more heavily scented at night to attract night-flying moths. As an aside, whilst some excellent examples of Jasmine sambac absolute and various Orange flower qualities exist in the AT marketplace (viz: concrete, absolute and the hexane extracted water distillate of Orange flower called Eau de Bruts), some Gardenia and Honeysuckle qualities sold to gullible Natural Perfumers and Aromatherapists prove to be fragrance compounds, so be careful!
Artifacts and Distilled Lime Oil.
According to Mans
Boelens (1997) artifacts are the fourth category of substances influencing the
odour of essential oils - the remainder being character compounds, essential
(non-character) compounds, and balance (non-essential, non-character) compounds.
Perhaps there is no better an example of artifact formation in essential oils
than that afforded by distilled lime oil production from Key limes (Citrus
aurantia), where slurries of crushed lime fruit are produced by screw
pressing, and the naturally acidic pulp lime juice-peel mix is charged to an
alembic, where it is distilled for 8-10 hours at a pH of 2-2.5 and temperature
of 96-98°C,
the essential oil being condensed and separated in the normal way. Distilled
lime oil, by the way, is the only distilled citrus oil that has found a
substantive market-slot – other distilled citrus oils are pale olfactory
shadows of the original pressed oils.
With apologies, here is
a technical paragraph to explain what happens chemically during lime oil
distillation. Clarke B.C. & Chamblee T.S. (1992) discuss the differences
between expressed and distilled lime oils, considering that the rapid reactions
of the bicyclic monoterpenes a-pinene,
b-pinene,
sabinene and thujene under acid conditions accounted for the major differences,
forming a-terpineol,
terpinolene. a-fenchol,
borneol, a-terpinene,
a-fenchene,
camphene, g-terpinene,
terpinen-4-ol and limonene as primary products. Products like g-terpineol,
b-terpineol
and terpinen-1-ol were suggested to have been formed from limonene, pinenes or
terpins, whilst elevated 1,4- and 1,8-cineol levels were proposed to stem from
limonene under milder acid conditions. 2,2,6-trimethyl-6-vinyltetrahydropyran
and 2-(2-buten-2-yl)-5,5-dimethyltetrahydrofuran are also known to result from
acid rearrangement of linalol, and contribute to the distilled lime odour.
So consequentially from
the above, distilled lime oil is therefore rich in alcohols, ethers and ketones
but poor in aldehyde- and bicyclic monoterpenes compared with the expressed oil.
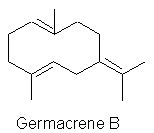
Finally, Clark (1987) found that differentiation of lime from lemon oil aroma
owes much to the presence of Germacrene B, with its woody spicy geranium like
odour, and was described as an impact odour constituent of the expressed oil.
Clark B.C. & Chamlee T.S. (1992) consider than Germacrene B is not found in
distilled lime oil because of its’ heat lability (rearranging to form elemene
and selinenes).
Although the above situation is a somewhat exaggerated example, similar rearrangements of odourous principles of fruits, flowers, leaves and barks occur in steam distillation, where the pH often drops during the course of the distillation process.
Minor Essential Oil Constituents with a Big Impact.
Some odiferous
components of essential oils are present in quantity i.e. in many Helichrysum
oils it is the levels of g-curcumene
and a-curcumene
which are important in creating the “curry” notes (these can be present at
up to 20% each in some chemotypes of H. italicum subsp. italicum
from the Adriatic coast). In other cases, very minute amounts of a powerful
odourphore can dominate the profile. In
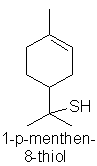
grapefruit oils from Citrus x paradisi cultivars,
for example, the compound known as
Grapefruit thiol (p-1-menthen-8-thiol) typically present at 1 part per billion
in grapefruit oil lends an instantly recognizable grapefruit juice tang to the
oil (although other minor constituents such as ethyl butyrate, (Z)-3-hexenal,
1-hepten-3-one, 4-mercapto-4-methyl-2-pentanone, wine lactone are also
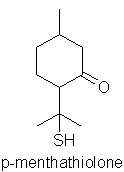
important). A similarly constructed ketone: p-menthathiolone contributes to the
fruity sulphurous odour of Buchu leaf oil, together with a number of other
sulphur compounds, despite their low concentrations in the oil.
Aldehyde C-8 (1-octanal) is important to the citrus note of lemon oil in
contrast to its occurrence at 0.1%. Many substituted pyrazines, thiazole and
pyridine compounds occur in trace amounts in coriander oil from Coriandrum
sativum, individually possessing roasted, nutty, earthy and spicy character
aspects, and being indispensable to the overall character of the oil.
It is of course amusing to speculate that certain aromatherapeutic properties of essential oils may owe more in certain instances to the minor character impact materials than to the major components, especially where psychophysiological processes such as subconscious recognition, and perhaps hedonistic preference and familiarity rely on the presence of these substances.
Stereochemistry and Odour.
Understanding the influence of the enantiomeric forms of certain naturally occurring substances in essential oils, is part of the puzzle in understanding the perception of odour quality and authenticity of those oils. This is related to the fact that different enantiomers of optically active oil constituents can have differing odour profiles. Familiarity with the odour profiles of authentic oils provides us with a mental reference point (odour memory) of the typical oil, in which these ratios of naturally occurring isomers are firmly set. Further, we can talk about the enantiomeric purity of a naturally occurring isomer in an authentic essential oil, which will normally fall between fairly tight margins. For example lavender oil (Lavandula angustifolia) might normally contain 99% optically pure R-linalol, but if distilled for a long time at pH5, this might drop to 93% minimum (H. Casabianca et al. 1998). The typical odour of lavender oil is due in some part to this relatively optically pure form of linalol, and in the relative optical purities of all the other chiral constituents Therefore an analytical technique which can establish the enantiomeric purity of these isomers (chiral gas chromatography) can be used to establish authenticity of well-characterised oils from certain defined geographical locations.
As many synthetic substances contain equally balanced mixtures of enantiomers and are thus racemic, these synthetics will often differ in odour characteristics from non-racemic (chiral) substances contributing to the odour profile of an essential oil. It is often therefore possible for the “trained nose” to detect the adulteration of essential oils with an added coupage of a major component e.g. Bergamot oil expressed, with added synthetic linalyl acetate, may be detected by an analyst in simple blind triangular QC test procedures, even before submitting to instrumental methods of detection. Adulterers are resourceful however, and in the example above, rather than use synthetic linalyl acetate, the more desirable “natural” laevo linalyl acetate can be cheaply obtained as acetylated Ho oil….. and so the cat and mouse game of spotting adulteration continues!
The human nose is unable to tell the chiral forms of camphor apart, but does significantly better with these other enantiomers (M. Boelens 1993):
Carvone R-(-) fresh herbal, spearmint-like
Carvone S-(+) fresh herbal, caraway-like
Limonene R-(+) fresh, natural, citrusy, orange-like
Limonene S-(-) harsh, turpentine-like, lemon note
Linalol R-(-) flowery-fresh, reminiscent of lily-of-the-valley (Hmm. I would
say lavandaceous, woody! – TB)
Linalol S-(+) differs slightly. (I would say sweet, more lavender-rosaceous
– T.B.)
Menthol 1R, 3R, 4S-(-) refreshing, strongly minty, cooling
Menthol 1S, 3S, 4R-(+) minty, musty, phenolic, medical
a-Pinene
1R, 5R-(+) harsh, terpene-like, slightly minty
a-Pinene
1S, 5S-(-) harsh, terpene-like, coniferous
a-Terpineol
R-(+) heavy, floral, lilac-like
a-Terpineol S-(-) tarry, coniferous, cold pipe-like
Boelens MH et al (1993) Perfumer & Flav. 18 (Nov/Dec) p1
Boelens, M (1997) “The differences in Chemical and Sensory Properties of Oreange Flower and Rose Oils from hydroduatillation and supercritical CO2 extraction Perf & Flav. 22 (May/June 1997) p31.
Burfield T. (2002) “Odour Profiling of Essential Oils” In Essence 1(2), 14-18.
Burfield T. (2002a) Natural Perfumery Notes: Module One. pub. AIA UK 2002.
Casabianca H. (1998) “Enantiomeric Distribution Studies of Linalol and Linalyl Acetate” J. High Resol. Chromatogr. 21, (Feb 1998) p108-111.
Clarke B.C. (1987) “HPLC Isolation of the Sesquiterpene Hydrocarbon Germacrene B from Lime Peel Oil and Its characterisation as an important Flavour Impact Constituent” J. Agric Food Chem. 35, 514-518.
Clarke B.C. & Chamblee T.S. (1992) “Acid-catalysed Reactions of Citrus Oils and Other Terpene-Containing Flavours” in Charalambous (ed) Off-flavours in Food & Beverages pub Elsevier p229 et seq.
Clarke BC & Chamblee T.S. (1997) “Analysis and Chemistry of Distilled Lime Oil (Citrus aurantifolia Swingle)” J of Essential Oil Research 9(3), 67-274
Kaiser R. The Scent of Orchids Editions Roche 1993.
Kaiser R. (1997) “Environmental Scents at the Ligurian Coast” Perf. & Flav. 22(5), 7-18.
King J.R. “Aromatherapy from the Hills” Psychiatry in Practice Spring 1998 p20.
Lamparsky 1987 “Fingerprints in Essential Oil Analysis – Aim, Results, and Correlations with Sensory Values” in Capillary Gas Chromatography In Essential Oil Analysis ed P. Sandra & C. Bicci pub Huethig NY 1987).
Mookerjee (1989) Aerosol Age May 1989 pp20-23 & pp 44-45
Morales R. (1986) “Taxonomia de los generos Thymus (excluida la seccion
Serpyllum y Thumbra) en la Peninsula Iberica” Ruizia 3, 7-11.
Tisserand R (1985) The Essential Oil Safety Data
Manual pub. Tisserand Aromathertapy Institute, Brighton.
Pandey G (2000) Medicinal Plants of
the Himalaya Vol 2 Ind. & Oriental pub. Delhi 2000.
Alkyl group: A chemical
group attachment built from an alkane, from which a hydrogen has been extracted.
.
Artifacts: unnatural substances that can be formed by man’s
intervention i.e. when trying to capture the essence of a flower by
distillation. These compounds may be seen as
either beneficial (e.g. chamazulene formation during distillation of Chamomile
oil), or undesirable.
Cis-isomer:
a geometric
isomer where the groups are on adjacent sides of the double bond.
Chiral substances:
the ability of
a specific substance to occur as 2 or more optical isomers. Individual
enantiomers (q.v.) often have different odour profiles e.g. (-)- carvone is
minty, (+)-carvone is caraway-like.
Enantiomer: an individual optical isomer (see chiral substances).
Headspace: the enclosed airspace above an odour source; the latter might be a flower or surface of a liquid. Headspace odourants may be collected for analysis by sweeping the fragrant airspace with an inert gas onto an absorbent material (such as a Tenax® trap, where the absorbed odours can be later liberated by thermal desorbtion onto a GCMS for subsequent analysis), or by solvent- or freeze-trapping etc. Advantages of headspace collection include the simplicity of the technique, and a reduction in the likelihood of artifacts being produced by thermal degradation – which makes it very suitable to collect the thermolabile odourants from certain flowers. Drawbacks of the technique include the low volume of collected material that can be realised and the generation of artifacts by non-thermal routes*.
Isocyanates: A group of compounds frequently
occurring in the Cruciferae,
often reponcible for spicy or irritant nature of some species i.e. phenylethyl
isocyanate in the horseradish Armoracia
rusticana.
Littoral:
pertaining to the sea-shore area.
Nitriles:
a compound containing a sp hybridised nitrogen
atom linked to a carbon atom by a triple bond. Common in the living volatile
odours of flowers.
Trans-
isomer:
a geometric
isomer where the groups are on the opposite sides of the double bond.
*From T. Burfield (2000) Natural Raw Materials – Odours & Origins
edn 1. pub. AIA (Tampa).
Copyright Tony Burfield Jan. 2003.