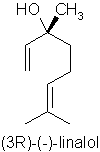
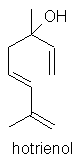
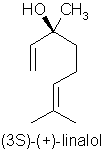
Substituting for Rosewood Oil Aniba rosaeodora var. amazonica Ducke – a look at other high linalol containing oils.
[Adapted and modified from an article for Aromatherapy Today Vol 26, June 2003 pp30-37, and reproduced by kind permission of the editor, John Kerr].
The demise of the natural Rosewood populations (from Aniba spp. Aublet
Fam. Lauraceae)* in Brazil is well known, due to over-exploitation for essential
oil-producing purposes to supply the perfumery trade. Wild stands of trees still
exist in deep forest areas, but poor or absence of regeneration in exploited
areas, and disappointing silviculture growth trials, fuel continuing concerns.
Leaf oil trials offer a hope of future sustainable oil production policy (FAO
2002) but are probably a long way from commercial reality, and anyway have been
very a long time coming, being mentioned forty years previously by Arctander
(1960). Loss of germplasm diversity and a narrowing of the genetic base are now
being addressed for Aniba spp. with moves to establish a germplasm
collection; an ‘Adopt a tree’ campaign has been instigated by Dr. Manuel
Limas of Manaus University, details of which can be viewed on www.ifaroma.org.
The latter, although commendable, may prove an ineffective gesture in the light
of deforestation information obtained by satellite monitoring from the Brazilian
National Institute of Space Research which shows a 40% rise in Amazonian tree
felling in the past year (The Independent 2003).
Part of a proposal by
the Botanical Medicinal Academy (BMA) recommends investigation of the use of
substitute plants for endangered or threatened plants (Yarnell E. & Abascal
K. 2001). In the absence of any lead in this direction from any professional
alternative health organisation, and with the BMA’s policy in mind, the
properties of Brazilian rosewood oil (Aniba spp.) are now considered, and
possible suitable replacement oils are examined.
*Do not
confuse with Dalbergia nigra, which is also called Brazilian Rosewood,
but is a hardwood used (amongst other things) for musical instrument
manufacture. Rosewood oil from Aniba species is also known as bois de
rose oil, or bois de rose femelle.
Historical sketch
The
quest for sources of fragrant raw perfumery materials such as the monoterpene
alcohols geraniol, citronellol and nerol, originally prompted essential oil
distillers to derive them from common essential oils, either because there was
no alternative economic synthetic route, or the available synthetics were of
inferior odour value to the derived materials. When the author (TB) first
entered the aroma business, one of my first experiences in the trade was to
prepare the perfumery raw material “rhodinol” from the saponification of
terpeneless Geranium oil Bourbon fractions (Pelargonium graveolens), to
produce the mixture of natural alcohols which include geraniol and laevo-citronellol,
and which typically had a “red rose” note.
Linalol
was another sought after perfumery ingredient, and to satisfy market demand,
companies would saponify essential oils containing linalyl esters, such as
Bergamot (Citrus aurantium subsp. bergamia) & Petitgrain (Citrus
aurantium subsp. aurantium). Linalol ex Lavender oil (Lavandula
angustifolia) is still available commercially to this day, from certain
selected producers. Linaloe oils (Bursera spp. see below) from Mexico and
India were also used for linalol production, but poor attention to quality by
overseas producers resulted in market buyers looking elsewhere. The preferred
linalol source quickly became the distillation product of the comminuted wood of
the felled Amazonian tree
Pau-rosa (“rosewood” or “bois de rose”) from species of A. rosaeodora A.
Ducke, A.
rosaeodora var. amazonica Ducke
and other Aniba spp. such as A. amazonica A. Ducke and A.
parviflora Meissner Mez (Fam. Lauraceae).
All
about Linalol
If
you’ve ever smelled commercial synthetic 97% pure or 98% coeur grades of
linalol, or moreover, have compared them with a 99.99% ultra-pure synthetic
grade of linalol, you will have noticed the detracting smell of traces of
impurities such as dehydrolinalol, tetrahydrolinalol and perhaps linalol oxides
etc. Additionally, the enantiomeric purity of linalol has an effect on the odour
profile. Pure laevo-linalol is woody and lavender like, whereas pure dextro-linalol
is sweet coriander-like; pure (synthetic) racemic linalol is somewhere in the
middle of the two. Further, linalol isolated from an essential oil e.g. linalol
ex bois de rose, may have traces of other oil components, which add to and
colour its odour profile, such as para-methyl acetophenone, 3-octanol, methyl
heptenone etc. These trace items help to modify the profile of pure linalool,
giving to a more “authentic” rosewood note. The linalol contents of some
common linalool-containing oils are shown below (Table 1) with the enantiomeric
purities of the contained linalol set out in Table 2.
|
Oil |
%
Linalol content |
|
Coriander |
60-80 |
|
Kuromoji |
50-70 |
|
Ho
Wood |
50-75* |
|
Linaloe
Mexican |
60-70 |
|
Linaloe
Indian |
40-75 |
|
Rosewood
Brazil |
65-90 |
|
Sweet
Basil |
45-62 |
|
Mentha
citrata |
20-50 |
|
Lavender |
30-35 |
|
Petitgrain |
20-30 |
|
Bergamot |
10-30 |
|
Skimmia
laureola |
17.5% ** |
Table
1. Linalol
content of selected essential oils.
*Zhu
et al. (1994) ** Misra PN et al.
2000
|
SD
Oil |
Enantiomeric
excess |
|
|
|
R |
S |
|
Bergamot
|
100 |
0 |
|
Ho
China |
97.6 |
2.4 |
|
Petitgrain |
72.2 |
27.8 |
|
Lavender
French |
96 |
4 |
|
Coriander |
10 |
90 |
|
Rosewood |
50-51 |
49-50 |
Table
2. Enantiomeric
ratio figures for linalol in selected essential oils.
[Adapted
from Casiabanca H. et al. (1998)]
Brazilian
Rosewood Oil
When
cheap synthetic racemic linalol became more commonplace in the 1960’s, linalol-containing
oils could be used in their own right for their unique odour profiles. It is
widely reported that Rosewood oil has a history of use in upmarket fragrances -
Chanel No. 5 (Chanel 1921), Aromatics Elixir (Clinique 1972), Ungaro Pour Homme
III (Ungaro 1993) all contain rosewood oil, as more recently, does Sensi by G.
Armani – these upmarket uses now perhaps contrast with its former use in cheap
soap perfumes.
To produce rosewood oil, slow-growing rosewood
trees (which reach 30m.) are felled, and after cutting into 1m. sections are
transported down-river, or larger sections are floated there. On arrival at the
distillery the wood is chipped and distilled (usually in a 1000 Kg chip capacity
mobile still) – the yield is typically in the region of 1%. Over-exploitation
has meant that rosewood stands are now becoming more remote from established
distilleries. Mors & Rizzini (1966) report that rosewood trees were found in
the areas of Juriti Velho and Maués, and along the Jamundá and Oyapoc rivers,
estimating actual annual production at 300-400 tons (twice the quoted official
figure), although its range includes the countries of Peru, Colombia, Ecuador,
Suriname and French Guiana, as well as areas of Brazil. Now (2003) it is
estimated at less than one tenth of this figure, at 20-30 tons.
Ohashi
S.T. et al. (1999) report on the scope for sustainable production of rosewood
oil from Curua Una in Brazil, and present analyses of the wood and leaf oil
compositions found in their trials, and contrast them with commercial wood oils.
The authors were able to show that the enantiomeric composition of oil from
different trees varied according to inherited genetic factors. Some GC/MS
analysts take the eremophilene content as an index of quality of the wood oil,
but since rosewood leaf oil contains relatively higher amounts of this compound,
maybe this offers a possible ‘adulterers charter’ to mix the leaf oil with
synthetic linalol, and pass it off as Rosewood oil. Wholesale adulteration of
the wood oil occurs already however, adulterated oils being termed “US
quality” in the trade, as the only unadulterated oil is shipped directly from
Manaus, Brazil.
|
Ho leaf oil (Cinnamomum spp.) (Ohashi et al. 1997) |
Rosewood Curua Una leaf oil
(Ohashi et al. 1997) |
|
0.10% cis-b-ocimene
0.08% b-pinene 0.03% myrcene 0.04% limonene 0.16% 1,8-cineole 0.04% trans-b-ocimene 0.02% p-cymene 0.25% cis-linalol oxide 0.47% trans-linalol oxide 0.57%
camphor 97.47% linalol 0.05% terpinen-4-ol 0.33% hotrienol |
0.06% cis-b-ocimene
0.03% b-pinene 0.06-0.10% myrcene 0.10-0.15% limonene 0.03-0.04% 1,8-cineole 0.03-0.05% trans-b-ocimene 0.03% p-cymene 1.20-1.80% cis-linalol oxide 1.00 to 1.54% trans-linalol oxide 1.40-2.70%
cyclosatirine 73.00-78.00%
linalol 0.14-0.53% terpinen-4-ol 0.40-0.54% hotrienol 0.10-0.50% g-selinene 4.50-6.00% eremophilene 3.10-4.40% unknown 0.13-0.25% epoxy-linalol 0.04% linalol oxide D-pyranoid 0.80-0.95% caryophyllene oxide 0.35-0.62% spathulenol 0.62-0.79% benzyl benzoate |
Table 3. Leaf oil analysis of Ho and Rosewood leaf
oils. From (Ohashi et al. 1997).
Schultes & Raffauf (1990) review the Lauraceae
in S. America, noting synonymy between Aniba, Nectandra and Ocotea
species, and the rich occurrence of alkaloids in the group. Gottlieb &
Kubitzki (1981) have reviewed the chemistry of the forty Aniba spp.
Interesting to the authors is the statement by Arctander’s Perfumery and
Flavour Chemicals Vol. II where the toxic compound nitro-benzene is
described as having a odour similar to “certain lots of poor grade Bois de
Rose.” Arctander claims analysis of these oils reveals nitro-compounds closely
related to nitrobenzene. In particular Arctander speculates that
1-nitro-2-phenylethane might be responsible for an “unattractive shoe-polish
top note”, speculating further that it might be as a result of contaminated
drums or from co-gathering the leaves of Aniba caniella. [Aniba
caniella has a scented bark described as smelling of cinnamon & roses].
But we digress…
Cayenne bois-de-rose oil from French Guiana is now
rarely seen, but samples analysed in the 1990-1995 period by the author were
found to contain 80-97% linalol, and bear some resemblance to oils now traded as
S. American Ocotea caudata, especially in respect of isovaleraldehyde and
furfural contents.
Peruvian
Ocotea caudata samples seen by the author (TB) seem to be dominated by a
clean pine disinfectant note that seems to be related to the presence of a-terpineol,
an artefact probably arising from the cyclisation of linalol at low pH during
distillation. Casiabanca et al. (1998) additionally notes that the pH of
rosewood drops to 3.8 at the end of steam distillation, and suggests that pH
drop will lead to the partial racemisation of linalol enantiomers. This is of
importance for analysts deliberating on authenticity criteria for such oils.
|
Ocotea
caudata commercial
(T. Burfield unpublished data) |
Aniba rosaedora Brazil (T.
Burfield unpublished data) |
|
0.79%
isovaleraldehyde 0.59%
furfural 0.24%
camphene 0.20%
b-pinene ---
myrcene 0.64%
limonene 0.98%
1,8-cineole 0.02%
cis-ocimene 0.03%
tr-ocimene tr
n-octanol tr.
3-octanol 0.16%
camphor tr.
para-methyl acetophenone 1.01% cis-linalol oxide 1.21% tr-linalol oxide 87.5% linalol 0.08% terpinen-4-ol 2.84% a-terpineol 0.13% hotrienol 0.18% nerol* 0.40% caryophyllene oxide 0.23% spathulenol 0.98% benzyl benzoate |
0.26%
b-pinene ---
myrcene 0.16%
limonene 0.80%
1,8-cineole tr.
cis-ocimene tr.
tr-ocimene tr.
methyl heptenone tr ----
methyl heptenol tr.
3-octanol tr.
camphor 1.59% cis-linalol oxide 1.39% tr-linalol oxide 0.35% a-selinene
0.30% a-copaene 89.86% linalol 0.02% E,Z-2,6-dimethyl,
5,7-octadien-2-ol 0.02% linalol epoxides 0.21% terpinen-4-ol 2.61% a-terpineol 0.10% hotrienol 0.72% nerol* 0.16% geraniol 0.22% eremophilene 0.08% nerolidol 0.23% spathulenol 0.28% benzyl benzoate |
Table 4 South
American Rosewood Oils
(Tony Burfield – unpublished data)
* the geraniol isomer was expected
to dominate in these analyses, in accordance with the literature, with only
traces of nerol present, but was not found in these cases.
Mexican
Linaloe oil
Mexican
linaloe oil was produced from the wood and possibly the seed of Bursera aloexylon
and B. delpechiana species – perhaps also other inferior species (the
EOA standards also mention B. fagaroides var. ventrocosa when
setting the specification criteria for Linaloe wood oil, but this has been
subsequently questioned). Producing centers were in the states of Puebla and
Colima. Adulteration with inferior species, and with linaloe seed oil (giving a
more positive optical rotation), and which kept less well than the wood oil,
lead to a quality decline in the years after 1920 which in turn lead an
increased preference for Brazilian Rosewood Oil. The high yield (over 10% in
direct steam fired stills), the presence of 60-70% laevo-linalol and up
to 10% of linalyl acetate (and absence of camphor), together with methyl
heptenol and linalol oxides give a pleasing profile to the oil, which has a
lily-of-the-valley like quality, and was highly regarded by (Western) perfumers
of the day (Burfield 2003).
Indian Linaloe Oil
Bursera delphechiana, and
other Bursera spp. including B.
simaruba L. and B. aloexylon are used to
prepare linaloe oil. In India it is more often from the air-dried husks
or pericarp of the fruiting berries, which are collected from the ground,
occasionally from the leaves; the fruit oil is not sold separately as it keeps
poorly, but is mixed with the wood oil. Cultivation
occurs in Karnataka, Maharastra & Andrah Pradesh, where there is up to 800
hectares under cultivation.
Indian linaloe oil is colourless or pale yellow
oil, the odour strongly reminding of linalol and freshly ground coriander,
with much radiance. Rosewood oil is flatter in comparison (Burfield 2000). The
oil reportedly contains >75% linalol, with geraniol and a-terpineol
which heavily contribute to its odour (giving a slight rosaceous-lilac aspect),
although other analyses suggest 35-45% linalyl acetate and up to 48% linalol.
Whereas total production volume per annum of the Mexican oil is believed to be
approx. 300Kg; 50-60 tons of the Indian oil is produced: which is totally
consumed by India’s internal perfumery trade (Shiva et al. 2002).
Kuromoji
Oil
Kuromoji
oil is obtained by steam
distillation of the ovate-oblong leaves of the evergreen trees Lindera
umbellata Thumb., L.
membranacea Maxim and L. sericea
Blume, which have with grayish-green bark native to the mountain ranges in
Japan, specifically Honshu, Shikoku, Kyushu and also China. The oil was produced
in Izu as a linalol source during the Second World War. The oil itself is seen
as yellow liquid with a fresh odour reminiscent
of linalol, one of the principle components, with a hint of orange (Burfield
2000). The dry-out is
lavandaceous, slightly wood-herby, soapy. The oil typically has a negative
optical rotation of –11°
to -12°;
the fresh odour is due to the relatively large amount of 1,8-cineol in the oil;
it is not available in the quantities that would be required to substitute for
Rosewood oil.
Ho Oils
Ho wood oil is often a blend of essential oils
produced by the steam distillation of the wood of Cinnamomum species
such as Cinnamomum camphora L. var. linaloolifera
and Cinnamomum camphora Sieb var. glavescens
Hayata (Zhu et al. 1994) from China, and also Formosa & Taiwan. The more
sweet camphoraceous leaf and branch oils of these species which have linalol
content as low as 15% may have to be fractionated and rectified to produce oils
with a high laevo-linalol and negligible camphor content, although in a
different publication Zhu et al. (1993) report on leaf oils from species
containing 90% linalol. Leaf oils have competed with wood oils in the last
several decades, although the exact distinction can be obscure. And so
rectification, or double rectification of these oils, produces products often
marketed as rectified ho oils in qualities containing from 95% to 99.5%+
linalol content; these are sometimes simply traded as ‘laevo-linalol ex
Ho’. Leaf and stem
oils containing 97% laevo-linalol from Cinnamomum tenuipulis found
throughout S. Yunnan are also mentioned as potentially exploitable by Zhu et
al. A few months ago I would have said that ho wood and leaf oils have a
bright future. In recent times double rectified ho oil qualities offering over
99.5% laevo-linalol and <0.02% camphor and optical rotation values of
up to –17.0°
have been cheaper industrially at times than synthetic linalol coeur on the open
market. Acetylation of ho wood oil to produce acetylated ho oils (mainly
consisting of linalyl acetate, with some rearrangement minor amounts of other
esters), together with ho oil itself as a linalol source, has been known to find
its way into commercial lavender and bergamot oils from many sources.

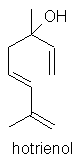
At the time of writing, with the Chinese
authorities introducing a ban on tree-felling of certain species including Cinnamomum
because of climatic concerns, ho oils have shot up in price. Zhu et al.
(1994) had already previously warned of potential problems of exhaustion of Cinnamomum
species reserves as no policy of tree replanting currently existed. To the
authors, the future sustainability of this commodity is unforeseeable at
present.
Coriander Oil
Coriander seed oil, CO2 extract and
oleoresin from the ripe seeds of Coriandrum sativa L., are familiar spicy
food flavouring ingredients. Coriander seed oil was originally important as one
of the few richer sources of natural dextro-linalol (aka coriandrol), but
its’ price has collapsed in recent years, and has, on occasion, even dipped
below the price of synthetic linalol. Post 2000, and especially in 2002,
coriander oil prices rose again and adulteration with synthetic linalol (and
even ho oil (!), which has a deleterious effect on the aroma profile) once again
becomes widespread and economic.
Petitgrain oil Terpeneless
Petitgrain oil terpeneless from Citrus
aurantium subsp. aurantium by fractionation has been passed off as
Rosewood oil to unsophisticated oil consumers in the past, but the distinctive
odour of linalyl acetate should give away its’ true identity to the more
discerning nose. Although terpeneless oils were favoured by the “father of
Aromatherapy”, Gattefossé, fractions of essential oil oils do not find favour
in mainstream aromatherapy practice, and so this matter is not pursued further.
For completeness, the enantiomeric distribution of linalool in authentic
petitgrain oil was determined as (3R)-(-)-linalol: 53.4-64.9% : (3S)-(+)-linalol
(35.1-46.6%) by Bernreuther and Schrier (1991), in contrast to the high
enantiomeric excess for (3R)-(-)-linalyl acetate (97%) : (3S)-(+)-linalyl
acetate (3%) Mosandl and Juchelka (1997).
Other Oils
The odour of oil from the leaves of Skimmea
laureola Sieb. & Zucc. ex Walp., often grown as an ornamental in
European gardens, has been likened to that of Petitgrain oil. The oil does
contain a modest proportion of linalol, but is dominated by high linalyl acetate
content.
The well-promoted Australian oil known as
“Rosalina”, is steam or water distilled from the terminal branches of wild-harvested
material of Melaleuca ericifolia
Smith, an evergreen shrub with a cork-like bark found growing in an area
stretching from Tasmania to New South Wales. Two chemotypes are known, a
1,8-cineole chemotype in the Southern parts of its natural range, and a linalol
type, which contains up to 55% linalool, to the North of the range. The
composition of the oil has been compared to tea tree oil excepting the major
constituent is linalol (Brophy et al. 1998), who gave the linalol content as
from 23-40%, with cineol (5-26%) and terpinolene (5-25%) also present in major
proportions. The oil inevitably also contains a-terpineol
from the rearrangement of linalol during distillation, and small amounts of
terpinen-4-ol. The odour of the linalol chemotype is camphoraceous and linalolic with
a medicinal quality and with some suggestion of the cleaner notes of tea-tree
oil. Realistic sustainability data for the species following any potential large
volume production of this oil is not clearly known, as far as the authors can
establish.
Background: the Physiological effects of differing linalol enantiomers.
Are
we able to foretell the physiological properties of high linalol-containing oils
from considering the physiological properties of linalol enantiomers?
Peana et al. (2002) noted the occurrence of
various isomers of linalol and linalyl acetate in various essential oils, and
investigated the anti-inflammatory properties of pure (-)-linalol, racemic
linalol and linalyl acetate, using carrageenan induced edema in rats as an
inflammation model - unfortunately (+)-linalol isomers were not included in the
study. [It is a great shame that we continue to see, and have to report on,
unethical testing procedures, considering the current global climate against
animal testing, and the moves to bring this to an eventual end within the EU - authors].
Although both forms caused edema reduction, at low levels of administration
(25mg/Kg), (-)-linalol delayed odema onset and reduced its effect, whereas the
racemate only reduced the effect. At higher levels no difference in effect was
seen between types. The effect of linalyl acetate on edema was delayed and
typical of pro-drug behaviour.
Further work by Peana et al (2003) involved
studies on the antinociceptive activity of (-)-linalol in mice using two pain
model techniques, acetic acid induced writhing and the hot plate test. In
addition the effect of applied concentrations of (-)-linalol @ 25, 50, 75 and
100 mg/kg on spontaneous locomotor activity was evaluated – here a dose
dependent increase in motility was observed eliminating a sedative
effect, and superficially at least, making an interesting comparison with
Buchbauer’s earlier findings (see below). Better performance of (-)-linalol
administration in the writhing
test over the hot plate test is consistent with the previous findings that (-)-linalol
possesses anti-inflammatory activity.
The authors suggested that (-)-linalol-induced antinociception
crucially involved participation of opioidergic and cholinergic systems.
Earlier
Atanassova-Shopova et al. (1973) had shown that linalol and terpineol (no
isomers stated for either compound) possessed CNS depressive effects and the
depressive effects of lavender oil were
partly at least but not totally due to these compounds. It should be stated that
the lavender oil used in the experiments had an unusual high (15.2%)
“terpineol” content. Much other work, too extensive to comprehensively
review, has been done on CNS effects of lavender e.g. neurosedative tests on
mice fed lavender oil in olive oil 1:60 (Guillemain et al. 1989),
anti-convulsive effects in mice inhaling lavender oil vapour (Yamada
1994), and my favourite, stress reduction caused by lavender straw as indicated
by reduction of travel sickness in pigs (Bradshaw
et al 1998)! Buchbauer et al. (1993) looked at the effect of inhaled
compounds on the motility of normal and intraperitoneally applied caffeine
agitated mice. Most effective at decreasing motility in normal mice were
lavender and neroli oils (details of botanical origins missing throughout), and
the fragrance chemicals linalol*, linalyl acetate* and citronellal*. Caffeine
agitated mice were compensated best by lavender oil, isoeugenol*, linalol*,
linalyl acetate*, maltol, methyl salicylate and carvone*, but linden blossom and
neroli essential oils, methyl anthranilate and farnesol* increased the agitation
level and were perceived as stimulating. Mount Blanc lavender oil was noted as
being the most soporific of all substances tested. Overall, relatively low
concentrations of fragrance chemicals in blood caused sedative effects, effects
being achieved via direct action on cell membrane lipids in the cortex.
Buchbauer maintains that esters (such as linalyl acetate) are more lipophilic
than monoterpenols (such as linalol) and more easily pass the blood-brain
barrier and shows a table showing figures for inhaled esters and corresponding
monoterpeneols, some of which confirm this view.
*
isomeric composition not given
Manley (1993) had in the same year,
investigated a number of oils (basil, bergamot, rosewood, chamomile, clove,
geranium, lemon, lemongrass, marjoram, neroli, patchouli, peppermint, rose,
sage, sandalwood and valerian) by contingent negative variation and found that
their effects (sedative, stimulant or neutral) largely corresponded to their
traditional aromatherapeutic labelling.
Sugawara (1997) produced what appears to be
a seminal study in methodology for evaluation of the physiological consequences
of fragrance (essential oils) inhalation, relating odour effectiveness to work
type. Fragrance impression was scored under thirteen impression adjectives, and
scores of impression were taken on inhalation before and after work, and
subsequently statistically examined for significance of difference. Work could
either be a mental, physical or auditory experience (“hearing environmental
sound”). Effectiveness of fragrance for work was statistically evaluated in
terms of numbers of significant items as shown by student’s t-test
examination.
Lavender,
rosemary, peppermint, marjoram, cardamom, sandalwood, basil, lime essential oils
and linalol gave a more favourable impression after, rather than before, work.
Linalol, peppermint and lime performed unfavourably after being compared with
impression before work. Basil, linalol, peppermint and sandalwood after hearing
environmental sound gave a more favourable impression.
Basil gave a more favourable impression with mental work.
Part
of the study was involved with measuring forehead electroencephalographic
measurements after inhalation, where it was noted that linalol caused a greater
decrease of beta-waves after work, whereas mental work with agitated inclination
increased beta-waves. Experiments showing the sedative properties of linalol
using optically active R-(+)-, S-(-)- and RS-(+/-)-* [i.e. racemic] grades of
linalol showed that RS-(+/-)-linalol and (S)-(-)-linalol caused a decrease,
whereas (S)-(+)-linalol with agitated inclination was associated with an
increase of magnitude of beta waves.
Re L. et al. (2000) have considered further
the linalol containing oils which affect the CNS, including hypnotic,
anticonvulsant and hypothermic properties ascribed to these oils, which the
authors relate to a local anaesthetic effect of linalol (Ghelardini et
al. 1999 had previously demonstrated this in vitro and in vivo for linalol and
linalyl acetate, speculating on a antimuscarinic explanation or Na+
or Ca2+ blocking effect). Re et al. speculate on an inhibitory effect
of linalol on the acetylcholine (ACh) release and on the channel open time in
the mouse neuromuscular junction, which they were able to demonstrate (isomeric
linalol composition not stated).
Cavanaugh & Wilkinson (2002) produced
an interesting review of some 67 published papers on the biological activity of
lavender oil, pointing out that poor attention to methodology, botanical origin
and chemical analysis of the oils often hampers meaningful evaluation of
published evidence. Tisserand’s suggestion that neurological effects of
lavender are effected by enhancing GABA mediated effects in the amygdala, are
considered, as well as Re’s work on linalol above. Relaxant effects of
lavender are discussed in relation to Lis-Balchin and Hart’s anti-spasmodic
(1999) on animal smooth muscle, although the latter authors postulated an
intracellular cAMP mechanism rather than action via adrenergic or cholinergic
receptors, or yet any action via involvement of calcium or potassium ion
channels. The review seems to be on weaker ground when discussing analytical and
safety issues, but discussion of the anti-microbial activity of lavender oils
adequately covers many criticisms of poor methodology in essential oil
microbiology studies. Studies by Lis-Balchins et al. (1998) are cited as
examples by Cavanaugh & Wilkinson of current failure to find a relationship
between the anti-microbial activity and the major constituents of lavender oil
(such as linalol and linalyl acetate). It has to be remembered however that
these studies were made on commercial oils - no authenticity indicators (a-santalene
concentration, optical purity of linalyl acetate etc. etc.) are cited, and so it
may be that oil purity is an important variable, amongst others, in
anti-microbial efficacy. The authors conclude that from consideration of in
vitro MIC values – not too dissimilar to those of tea tree oil – that
lavender oil may be useful against topical infections and as a modulating agent
for general healing and post-infection recovery mechanisms.
The Aromatherapeutic properties of Rosewood and certain other high linalol-containing oils.
Odour quality. The Western perfumery
industry has never considered ho oils a satisfactorily performing substitute
ingredient for rosewood oil in high-class perfumery, but may, at least, have
used the oil in low cost perfumes. Zhu (1994) confirms the use of ho oil in
Chinese perfumery. Additionally Western perfumers have often preferred rectified
ho oil qualities for specific application to synthetic linalol. It is perhaps
pertinent to remind readers that fragranced retail products labelled as
“aromatherapy perfumes”, and constructed by classically trained perfumers
rather that aromatherapists, turned over £611 million sales in the UK the three
years during 1999-2002.
General properties
In aromatherapy education, rosewood oil does not feature heavily in EU &
US taught syllabuses. Although it is listed in ITEC courses (see below) it is
absent, for example, from the list of essential oils in the UK National
Occupational Standards for Aromatherapy Database list http://www.skillsforhealth.org.uk/db/default.asp# .
Ho and
linaloe oils do not feature in many aromatherapy texts, and in the US
aromatherapists only became familiar with these oils as possible “substitute”
for the “endangered rosewood” (SSH).
(a) Rosewood. The standard text book
for the ITEC aromatherapy courses (Tucker 2000) describes Rosewood’s
therapeutic actions as analgesic, antidepressant, antiseptic, bactericidal,
cytophylactic, cephalic, deodorant, stimulant & tonic, but the author offers
nothing in the way of direct clinical or medical proof of any of these actions,
but directly refers to Patricia Davis’s popular high-street book (Davis 1999).
Franchomme & Peneol (1990) cite, for the oils of A. parviflora and A.
rosaedora var. amazonica: anti-infectious, anti-bacterial,
anti-fungal, anti-viral, anti-parasitic, tonic and stimulant properties,
suggesting use in oral & bronchial infections of adults, children and
babies, vaginal candidosis, nervous depression, fatigue, and note its’
non-aggressive properties towards the skin and mucous membranes. Although some
796 references are included at the end of latter authors’ work, it is unclear
which, if any, specifically refer to these stated therapeutic claims. Balz
(1966) gives a list of properties very similar to that of Franchomme &
Penoel above, whilst Lawless (1995) manages to find a conference quote from 1906
in support of skin care properties. Valnet (1980) does not offer a monograph on
Rosewood oil.
Opdyke (1975) summarised the literature indicating
that Rosewood oil was reported to have anti-convulsant activity in mice &
rats, spasmolytic activity on isolated guinea pig ileum, and anti-microbial
properties amongst other properties. It was also reported as non-toxic,
non-irritating and non-sensitising.
Regarding in vitro microbiological studies
on Rosewood oil: Lis-Balchin (1994) found no anti-oxidant action for Rosewood,
found it potently active against 24/25 bacteria, but only 12 out of 20 Listeria
monocytogenes varieties. It was described as moderately active against three
out of three fungi; Maruzella (1958) previously found good action against five
out of five fungi. Lobato et al. (1989) examined a number of Amazonian oils
against Pseudomonas aeruginosa, Proteus mirabilis, Escherichia coli,
Edwardsiela tarda, Klebsiella pneumoniae, Enterobacter
aerogenes and Salmonella spp, however Aniba rosaedora oil was
only active against five of the above.
(b) Ho. Franchomme & Penoel (1990) list
for Cinnamomum camphora Sieb var. glavescens
Hayata: powerful anti-infectious, anti-bacterial, anti-viral and anti-fungal;
tonic, general stimulant, useful, against respiratory, digestive and genital
infections, asthenias.
Hili (2000) showed that ho leaf oil had a high
activity (as demonstrated by the inhibition zone technique for cultured
organisms) against the yeasts Saccharomyces pombe, S. cerevisiae,
and moderate activity against Candida albicans, Torula utilis, and
the bacteria E. coli and Staphylococcus aureus, and low activity
against Ps. aeruginosa. It should be noted that linalol and
linalyl acetate were already known to possess antibacterial and anti-fungal
properties according to Knobloch et al. (1989). In a further experiment Hili
carried out broth dilution assays on thirteen oils in DMSO including coriander
and ho leaf oils against the same micro-organisms above, noting some variation
between inhibition assay and broth assay. Coriander oil was slightly more
inhibitory than Ho leaf oil at a range of concentrations at concentrations from
100-500mg/ml,
and was more markedly anti-fungal than ho leaf, apart from against S. pombe.
Finally, and somewhat intriguingly, Hili also examined the anti-microbial
activity of 1,8-cineol, thymol, eugenol and linalol (no isomer stated). Whilst
the reduction in bacterial growth at 500mg/ml
against the same test organisms in broth culture was closely similar between
linalol and coriander oil, coriander was markedly more active against the yeasts
S. pombe, S. cerevisae and T. utilis.
(c) Coriander seed oil. Considering likely deodorant properties of
essential oils, Hili (2001) summarises the literature in noting that cinnamon,
clove, coriander and lemongrass are active against the Corynbacterium, Propionibacterium
and Streptococcal classes of bacteria associated with body odour
(although from a practical point of view, it should be noted that skin safety
was not taken into account). A number of studies investigate the anti-microbial
properties of coriander oil e.g. in vitro anti-fungal activity: Garg et
al. (1992).
Valnet (1980) lists coriander seed oil as having
internal uses as a carminative, stomachic, stimulant, formerly being held to be
an aphrodisiac and a memory aide, as well as external use as an analgesic.
Considerations &
Recommendations
It has been very difficult in this short study to
be able to adequately cover all the papers relating to the alleged beneficial
properties of linalolic oils. Extrapolating the findings of the findings of the
work of Pena & Sugawara, to our way of thinking, may give credence to the
view that the widespread practice of oil adulteration by the deliberate addition
of synthetics may change the expected physiological outcomes of aromatherapy
interventions using these oils (- excluding other influential factors such as
the therapist-patient relationship, patient expectations following intervention
etc.). Ironically however, in the case of rosewood, the addition of synthetic
racemic linalol to make ‘US quality’ rosewood oil may not actually detract
from its expected effects – as an analgesic, antidepressant, antiseptic, and
anti-bactericide! Addition of laevo-linalol (as ho oil) or racemic
linalol to the dextro-linalol rich coriander oil on the other hand would
be expected to make more substantial changes to its’ otherwise expected
therapeutic actions.
In
terms of finding direct substitutes for rosewood oil, high linalyl acetate
containing oils such as French lavender or bergamot (not considered here) would
appear not to offer odour similarity; the possibilities raised by the pro-drug
effects of linalyl acetate are intriguing, however. The widespread
recommendations of using ho oil as a substitute for rosewood oil are partly
endorsed for anti-inflammatory, antinociceptive and anti-bacterial purposes, but
some misgivings are perceived for anti-fungal purposes. We are in agreement with
some points raised by Cavanaugh & Wilkinson, viz.
that in-vitro microbiological testing results are not necessarily valid
indicators for human microbiological infections in client intervention
scenarios, and that the small quantity of literature reviewed on this subject is
not necessarily truly representative of properties of the oils examined.
Further, the mechanistic basis for microbial kill effects of linalol-containing
oils has not been considered here.
Finally, a cloud hangs over the continued unfettered use of linalol
containing essential oils in retailed products in Europe at least, as linalol is
listed as one of the 26 skin sensitisers in the 7th Amendment to the
EU Cosmetic Act, and is required to be labelled if present above certain
concentrations in cosmetic products (these may include certain aromatherapy
products – consult your professional AT organisation for guidance). Essential
oils commonly contain 16 of these 26 sensitisers, but as a group, are not,
apparently, to be treated as a special case in the regulations. Although workers
in the aroma field have maintained that there is a lack of scientific rigour in
published dermatological research in this area which has directly provided the
basis for the SCCNFP opinion underpinning this legislation (see relevant
sections of www.aroma-science.com) the
passing of the Act has already had a substantial negative effect on the
perfumery, natural perfumery and essential oil industries. Some industrial
customers have shied away from using natural raw products that necessitate
obligatory labelling warnings to customers, resulting in a sales decline for
certain sectors of the aroma business. This situation is a double blow for
natural raw material end-users – as we have seen Rosewood oil is threatened
both by genetic erosion in its natural habitat, and its substitutes are under a
legislative threat of erosion of unfettered freedom of use.
References:
Arctander S (1960) Perfume
and Flavour Materials of Natural Origin pub. Elizabeth NJ (USA).
Arctander S (1969)
Perfume & Flavour Chemicals II (K-Z) entries #2332 and #2336. pub. Elizabeth
NJ (USA).
Atanassova-Shopova S,
Roussinov KS & Boycheva I (1973) “On certain neurotrpoic effects of
lavender essential oil: studies on the effects of linalool and terpineol” Bull
Inst Physiology 15, 149-156
Bernreuther A. and Schrier P.
(1991) “Multidimensional Gas Chromatography/mass spectroscopy: A power tool
for the direct chiral evaluation of aroma compounds in plant tissues II linalool in
essential oils and fruits” Phytochem Analysis 2, 167-170.
Bradshaw RH et al. (1998)
“Effects of Lavender Straw on Stress and Travel Sickness in Pigs” .J of
Alt and Complem. Med. 4(3), 271-275.
Buchbauer C, Jirovetz L, Jager
W, Plank C, Dietrich H (1993) “Fragrance Compounds and Essential Oils with
Sedative Effects upon Inhalation.” Pharmaceut
Sc 82
(6), 660-664
Burfield (2003) from the
forthcoming 2nd edn. Natural Aromatic Materials – Odours &
Origins to be pub. by AIA.
Casiabanca
H. et al. (1998)
"Enantiomeric Distribution Studies of Linalol and Linalyl Acetate" J.
High Res. Chromatog. 21, 107-112.
Cavanagh
HMA & Wilkinson JM (2002) “Biological activities of lavender essential
oil.” Phytotherapy Res. 16, 301-308.
Davis,
P. (1999) Aromatherapy an A-Z CW Daniel, Saffron Walden, quoting The
Yearbook of Pharmacy & Transactions of the British Pharmaceutical Conference
(1907) p217.
EOA (1975) “Oil of Bois de
Rose Brazilian”. EOA No. 2. 3 pp. Essential Oil Association of USA.
FAO (1986) Aniba duckei
Kostermans. In Databook on Endangered Tree and Shrub Species and Provenances.
Forestry Paper No. 77. Rome: FAO. pp. 60-68.
FAO(2002) at http://www.fao.org/forestry/FOP/FOPW/NWFP/Digest/sent/6-2002.stm#P75_4850
Franchomme
P. & Peneol D (1990) l’aromathérapie excactment pub. Jollois.
Garg S.C. & Siddigini N.
(1992) “In vitrio antifungal activity of the essential oil of Coriandrum
sativum Linn”. J. Res. Ed. In. Med
. II(3) 11-13.
Ghellardini
C. et al. (1999) “Local
anaesthic activity of the essential oil of Lavandula angustifolia.” Plant
Med. 65,
700-703.
Gottlieb
OR & Kubitzki K (1981) Chemosystematics of Aniba. Biochem Syst
Ecol 9, 5-12.
Guillemain
J., Rousseau A & Delaveau P (1989). “Effets neurodépresseurs de l’huile
essentialle de Lavandula angustifolia Mill.” Ann. Pharmaceutiques
francaises
47(6), 337-343.
Hili
P (2001) The Antimicrobial Properties of Essential Oils Winter Press,
West Wickham Kent.
ISO (1976) Oil of rosewood, Brazil. International Standard ISO 3761-1976 (E). 2 pp. International Organization for Standardization.
Knobloch
K et al. (1989) J.Essen Oil Res. June 1989.
Lawless
J (1995) The Illustrated Encyclopedia of Essential Oils pub. Element,
Shaftesbury UK.
Lis
Balchin M., Deans SG, Eaglesham E. (1998) “Relationship between the
bioactivity and chemical composition of commercial essential oils” Flavour
& Fragrance J. 13, 98-104.
Lis-Balchin
M & Hart S (1999) “Studies on the mode of action of the essential oil of
lavender (Lavandula angustifolia P. Miller). Phytotherapy Res 13,
540-542.
Lis
Balchin M, Deans S. & Hart S (1994) Paper presented at 25th
International Symposium on Essential oils, Grasse, France.
Lobato
AM, Ribeiro A, Pinheiro MFS, Maia JGS (1989) “Antimicrobial activity of
essential oils from the Amazon” Acta Amazonia 19, 355-363.
Manley C H (1993)
“Psychophysiological effect of odor.” Crit
Rev Food Sci & Nutrition 33
(1):57-62
Maruzella
JC & Ligouri L (1958) J Am Pharm Assn 47, 280.
McCarthy M. (2003) “The
great rainforest tragedy” The Independent 28th
June 2003 p1.
Misra P.N., Hasan S.A. &
Kumar S. Cultivation of Aromatic Plants in India CIMAP 2000.
Morais AA, Rezende CMAM, Bulow
MVV, Mourao JC, Gottlieb OR, Marx MC, Rocha AI and Magalhaes MT (1972) “Oleos
essenciais de especies do genero” Aniba. Acta Amazonica 2,
41-44.
Mosandl A. and Juchelka D.
(1997) “Advances in the authenticity assessment of Citrus oils.” J Essen
Oil Res. 9, 5-12.
Opdyke
DLJ FCT, 16, 653.
Peana AT, D'Aquila PS, Panin
F, Serra G, Pippia P, Moretti MD (2002) “Anti-inflammatory
activity of linalool and linalyl acetate constituents of essential oils.”
Phytomedicine 2002, 9(8),721-6.
Peana AT, D'Aquila PS, Chessa
ML, Moretti MD, Serra G, Pippia P. (2003)
41 “(-)-Linalool produces
antinociception in two experimental models of pain” Eur J Pharmacol 460(1),
37-.
Pedroso, L.M. (1986) Silvicultura do pau-rosa (Aniba rosaeodora Ducke). pp. 313-324. In Simposio do Tropico Umido, 1, VII. Flora e Floresta. Belem: EMBRAPA-CPATU.
Re
L., Barocci S., Sonnino S., Mencarelli A., Vivani C., Paolucci G., Scarpantonio
A., Rinaldi L., Mosca E. (2000) Pharmacol Res 42(2),177-82.
Schultes R.E. & Raffauf R.F. (1990) “The Healing Forest” pub. Discorides Press, Portland, Oregan.
Shiva M.P. Lehri A., Shiva A
(2002) Aromatic & Medicinal Plants Intl. Book Distrib. Dehra Dun,
India.
Sugawara, Y. (1997) “Sensory
evaluation Method for Fragrance of Essential Oils” CCAB mini review – can be
seen in full on http://neo.pharm.hiroshima-u.ac.jp/ccab/2nd/mini_review/mr136/sugawara.html.
Tucker L. (2000) An
Introductory Guide to Aromatherapy ed Foulston J. Holistic Therapy Books.
Valnet J. (1980) ed. Robert Tissereand The Practice of Aromatherapy CW Daniel Ltd. Saffron Waldon UK.
Yamada
K. et al. (1994)
“Anticonvulsive Effects of Inhaling Lavender oil Vapour” Biol. Pharm.
Bull. 17(2), 359-360.
Yarnell E. &
Abascal K. (2001) “Dilemmas of Traditional Botanical Research” Herbalgram
55, 46-54.
Zhu L., Ding D. & Lawrence B.M. “The Cinnamomum species in China: Resources for the present and future” Perf & Flav. 19, July/Aug 1994, 17-22.
Glossary
Coeur:
A term describing a “heart cut” fraction from a fractional distillation
DMSO:
dimethyl sulphoxide
EOA:
Essential Oils Association
Pro-drug:
A substance which only has a perceivable physiological effect after modification
or bio-tranformation by the body e.g. for linalyl acetate, after trandformation
into linalool by body esterases.
MIC:
minumum inhibitory concentration
Racemic:
an equal mixture of enantiomers resulting in zero optical activity.
Saponification:
The alkaline hydrolysis of fatty acid esters, (such as citronellyl acetate) to
produce alcohols (such as citronellol).
Silviculture:
A branch of forestry related to the growing and tending of trees.